4 Steps for Successful IND Submission in Pharmaceutical Compliance

Overview
The article delineates four essential steps for a successful Investigational New Drug (IND) submission in pharmaceutical compliance:
- Thorough preparation
- Meticulous documentation
- Comprehensive compilation of application components
- Detailed review process
These steps are critical as they ensure strict adherence to FDA guidelines, significantly enhance the likelihood of approval, and facilitate a seamless transition through the drug development lifecycle. The importance of accurate documentation and regulatory compliance is underscored throughout the article, emphasizing the necessity for pharmaceutical professionals to engage with these compliance solutions.
Introduction
The journey from a promising drug candidate to a market-ready medication hinges significantly on the Investigational New Drug (IND) submission process. This critical step demands meticulous preparation and a profound understanding of regulatory requirements that can either facilitate or hinder a pharmaceutical development project.
As the stakes rise, sponsors must consider essential steps for a successful IND submission and how to navigate the complexities of regulatory compliance. By delving into these pivotal elements, this guide offers invaluable insights designed to enhance the likelihood of approval and streamline the pathway to clinical trials.
Understand the IND Submission Process
The IND submission process represents a pivotal milestone in the drug development lifecycle, necessitating a formal request to the FDA for permission to test an investigational drug in humans. This process encompasses several critical phases:
- Pre-IND Preparation: Prior to submitting an IND, sponsors must conduct thorough preclinical studies to collect essential data regarding the drug's safety and efficacy. Effective strategies for IND submission significantly enhance the likelihood of approval.
- Submission Requirements: Familiarity with the required documentation is essential, including FDA Form 1571, which serves as the cover letter for the IND request. Ensuring that all necessary documents are complete and accurate is crucial for a successful IND submission.
- Review Timeline: After presenting the application, the FDA has a 30-calendar-day period to evaluate it. During this time, sponsors are prohibited from initiating clinical trials, making thorough preparation in advance essential.
- Regulatory Compliance: A solid understanding of Good Manufacturing Practices (GMP) and other regulatory requirements is fundamental for a successful IND submission. AVS Life Sciences offers comprehensive services, including GMP audits, to ensure compliance with these standards, thereby facilitating the IND submission process and building trust with regulatory authorities.
Statistics indicate that approximately 70% of IND applications are approved on the first attempt, underscoring the significance of careful preparation and adherence to FDA guidelines. By mastering these foundational elements and leveraging AVS Life Sciences' expertise, sponsors can effectively navigate the complexities of the IND application process, ultimately enhancing their chances of successful drug development.
Gather Required Documentation and Information
Collecting the necessary documentation is an essential step in the IND filing process. Below is a checklist of crucial documents required for a successful application:
- The cover letter outlines the IND submission, which includes the drug name, intended indication, and study design. A well-crafted cover letter establishes the tone for the entry and emphasizes key elements of the proposal.
- FDA Form 1571 serves as the official cover letter for the IND submission, and it must be filled out precisely to guarantee adherence to FDA regulations. Recent updates to this form should be reviewed to align with current regulations.
- Investigator's Brochure (IB): This comprehensive document provides clinical investigators with essential information about the investigational drug, including its pharmacology, safety, and efficacy data.
- Preclinical Data: Safety and efficacy data derived from laboratory and animal studies are crucial to demonstrate the drug's potential for human use. This information must be robust and well-documented.
- Manufacturing Information: Detailed descriptions of the drug's formulation, manufacturing process, and quality control measures are necessary to ensure compliance with Good Manufacturing Practices (GMP).
- Clinical Study Protocol: A detailed plan outlining the study's objectives, design, methodology, and statistical considerations is essential for guiding the clinical trial process.
Ensure that all documents are meticulously organized and formatted according to FDA guidelines to facilitate a smooth review process. This preparation not only enhances the likelihood of approval but also aligns with the FDA's mission to protect consumer health and safety.
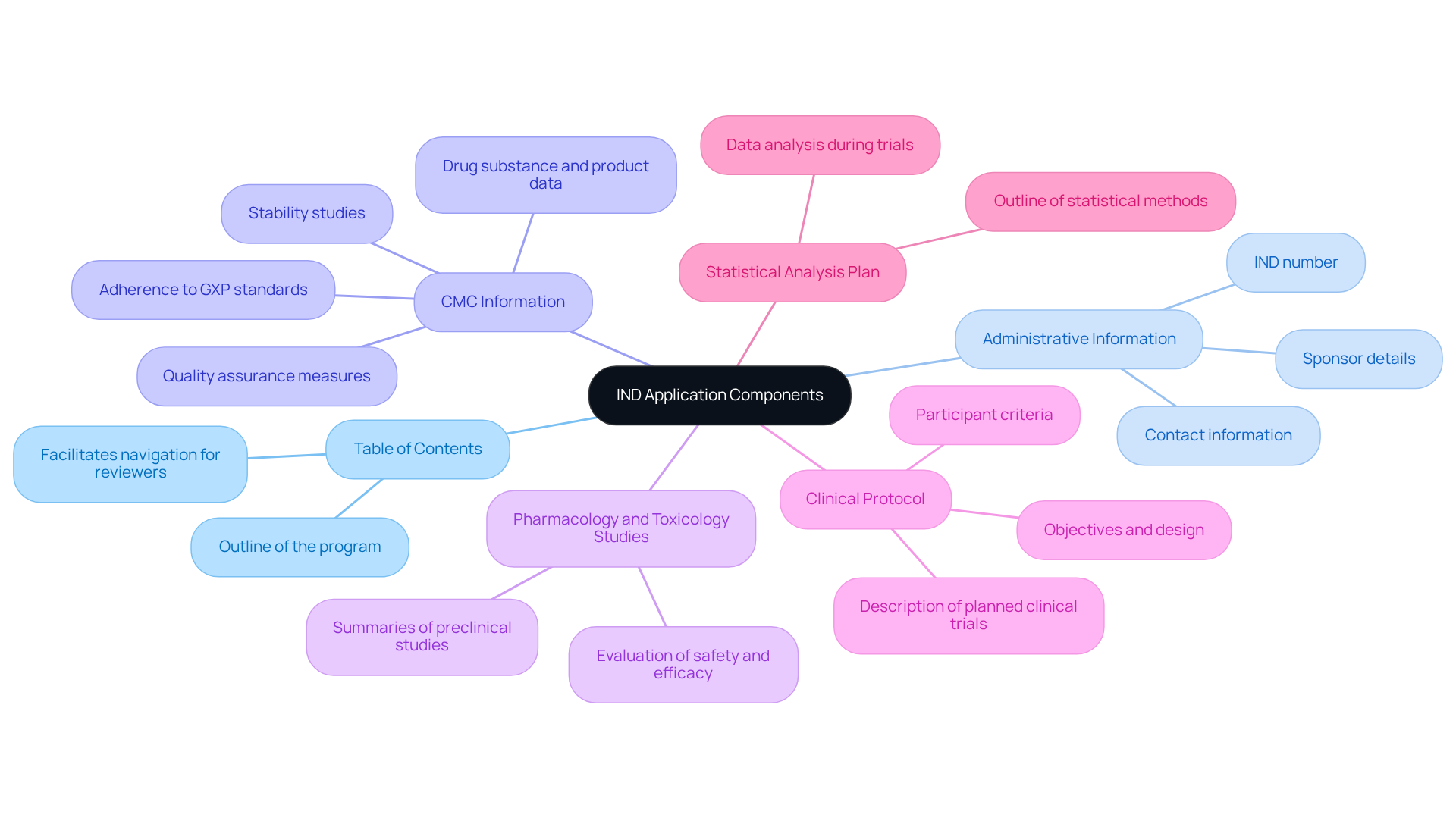
Compile Essential Components of the IND Application
Compiling the IND dossier necessitates assembling various components into a cohesive package that meets regulatory expectations. The key elements to include are as follows:
- Table of Contents: A clear outline of the program to facilitate easy navigation for reviewers.
- Administrative Information: Essential details about the sponsor, including contact information and the IND number.
- Chemistry, Manufacturing, and Control (CMC) Information: Comprehensive data on the drug substance and product, including stability studies and quality assurance measures. AVS Life Sciences underscores the critical importance of adhering to GXP standards and FDA regulations to ensure data integrity and compliance throughout this process, as evidenced by our successful upgrade of a biotechnology GMP facility.
- Pharmacology and Toxicology Studies: Summaries of preclinical studies that evaluate the drug's safety and efficacy.
- Clinical Protocol: A detailed description of the planned clinical trials, encompassing objectives, design, and participant criteria.
- Statistical Analysis Plan: An outline of the statistical methods that will be employed to analyze the data collected during the clinical trials.
By ensuring that all these components are meticulously included and well-organized, you significantly enhance the likelihood of a successful IND submission. AVS Life Sciences' proven excellence in quality management and regulatory compliance, highlighted by robust documentation practices and standard operating procedures (SOPs), can further bolster your efforts in navigating these complex requirements.
Review and Finalize Your IND Submission
Before the IND submission, conducting a meticulous internal review is essential to ensure accuracy and completeness. Follow these steps:
-
Internal Review: Assemble a dedicated team to evaluate the proposal for consistency, clarity, and compliance with FDA guidelines. This step is vital, as the FDA's Center for Drug Evaluation and Research (CDER) receives around 1,500 initial IND submissions each year, highlighting the competitive nature of the submission process. Ensure that all necessary documents and data are included to avoid common pitfalls that can lead to delays.
-
Proofreading: Carefully review the entire document for grammatical errors, formatting issues, and overall clarity. A well-presented IND submission not only reflects professionalism but also increases the chances of a positive evaluation.
-
Regulatory Compliance Check: Confirm that all components fulfill the FDA's regulatory standards, including proper formatting and adherence to protocols for filing. This step is critical, as compliance with Good Manufacturing Practices (GMP), ISO standards, and Quality System Regulations (QSR) is paramount in the life sciences sector.
-
Final Approval: Secure final approval from key stakeholders within your organization, including legal, regulatory, and clinical teams. This collaborative approach ensures that all perspectives are considered, reducing the risk of oversight.
-
Submission: Send the IND request through the appropriate channels, adhering strictly to the FDA's guidelines, including the required number of copies.
By meticulously reviewing and finalizing your IND submission, you can significantly enhance the chances of a successful IND submission and timely approval, ultimately contributing to the efficient progression of your pharmaceutical development efforts.
Conclusion
The successful submission of an Investigational New Drug (IND) application stands as a pivotal milestone in the drug development process, serving as a formal request to the FDA to commence human trials. This multifaceted process demands thorough preparation, meticulous documentation, and a profound understanding of regulatory requirements, all of which significantly bolster the chances of approval and pave the way for clinical development.
Key aspects of the IND submission process encompass comprehensive preclinical studies, precise documentation, and a well-organized application that aligns with FDA standards. By strictly adhering to the necessary guidelines, including the submission of critical documents such as the Investigator's Brochure and the Clinical Study Protocol, sponsors can markedly enhance the quality of their submission. Furthermore, conducting an exhaustive internal review prior to submission is crucial to ensure that all components are accurate and compliant with regulatory expectations.
Ultimately, the IND submission transcends being merely a procedural hurdle; it constitutes a fundamental aspect of safeguarding drug safety and efficacy for future patients. By dedicating time and resources to grasping the IND process, gathering the requisite documentation, and executing careful reviews, sponsors can adeptly navigate this intricate landscape. This proactive approach not only amplifies the likelihood of a successful IND submission but also fosters the advancement of medical innovation and public health.
Frequently Asked Questions
What is the IND submission process?
The IND submission process is a formal request to the FDA for permission to test an investigational drug in humans, representing a crucial milestone in drug development.
What are the phases involved in the IND submission process?
The IND submission process includes several critical phases: Pre-IND Preparation, Submission Requirements, Review Timeline, and Regulatory Compliance.
What is involved in Pre-IND Preparation?
Pre-IND Preparation requires sponsors to conduct thorough preclinical studies to gather essential data on the drug's safety and efficacy, which enhances the likelihood of approval.
What are the submission requirements for an IND application?
Familiarity with required documentation is essential, including FDA Form 1571, which acts as the cover letter for the IND request. All necessary documents must be complete and accurate for a successful submission.
How long does the FDA take to review an IND application?
The FDA has a 30-calendar-day period to evaluate the IND application after it is submitted. During this time, sponsors cannot initiate clinical trials.
Why is regulatory compliance important in the IND submission process?
A solid understanding of Good Manufacturing Practices (GMP) and other regulatory requirements is fundamental for successful IND submission, helping to build trust with regulatory authorities.
What services does AVS Life Sciences offer to assist with IND submissions?
AVS Life Sciences offers comprehensive services, including GMP audits, to ensure compliance with regulatory standards, thereby facilitating the IND submission process.
What is the approval rate for IND applications?
Approximately 70% of IND applications are approved on the first attempt, highlighting the importance of careful preparation and adherence to FDA guidelines.
