4 Steps for Facility Registration for Cosmetic and Skincare Manufacturers

Overview
This article delineates a four-step process for facility registration tailored specifically for cosmetic and skincare manufacturers. It underscores the critical nature of comprehending regulatory requirements, completing essential documentation, and ensuring adherence to Good Manufacturing Practices (GMP). By detailing pivotal steps such as:
- Preparing necessary information
- Accessing the FDA registration portal
- Tackling common registration challenges
It serves as a comprehensive guide designed to facilitate a seamless registration process. This approach not only addresses compliance challenges but also provides actionable insights that empower manufacturers to navigate the complexities of registration with confidence.
Introduction
Navigating the complex landscape of facility registration for cosmetic and skincare manufacturers is not just essential; it is crucial for compliance and success in a highly regulated industry. The introduction of the Modernization of Cosmetics Regulation Act (MoCRA) has made understanding the specific requirements set forth by the FDA more critical than ever. However, many manufacturers encounter significant challenges in this process. This raises an important question: how can they streamline registration while ensuring adherence to evolving regulations? This guide presents a comprehensive roadmap, empowering manufacturers to effectively tackle registration hurdles and maintain compliance with industry standards.
Understand Facility Registration Requirements
Before initiating the facility registration for cosmetic and skincare manufacturers, it is crucial to understand the specific requirements established by the FDA and other regulatory bodies. Key considerations include:
- Definition of Responsible Person: The Responsible Person can be the manufacturer, packer, or distributor of the cosmetic products. This entity is accountable for ensuring compliance with FDA regulations and must maintain records substantiating the safety of the products.
- Registration Necessity: Confirm whether your establishment must register under FDA guidelines. This involves assessing the scope of your operations to determine if they align with the definitions provided by the FDA. Under the , all cosmetic establishments are required to complete facility registration for cosmetic and skincare manufacturers and follow Good Manufacturing Practices (GMP).
- Documentation: Compile essential documentation, including proof of business establishment, facility address, and contact information. Precise and thorough documentation is essential for adherence and must be easily accessible for FDA inspections.
- Regulatory Updates: Stay informed about any changes in regulations, especially those introduced by MoCRA, which significantly affects compliance requirements. For instance, as of December 29, 2024, the labeling requirement for the Responsible Person will become mandatory, necessitating that their name, domestic address, and phone number be included on product packaging.
By fully grasping these requirements, manufacturers can steer clear of typical obstacles and promote a more seamless facility registration for cosmetic and skincare manufacturers process, ultimately enhancing their adherence and market preparedness. Furthermore, collaborating with AVS Life Sciences can offer expert advice and thorough solutions in quality management and regulatory compliance, ensuring that your establishment meets all necessary standards efficiently.
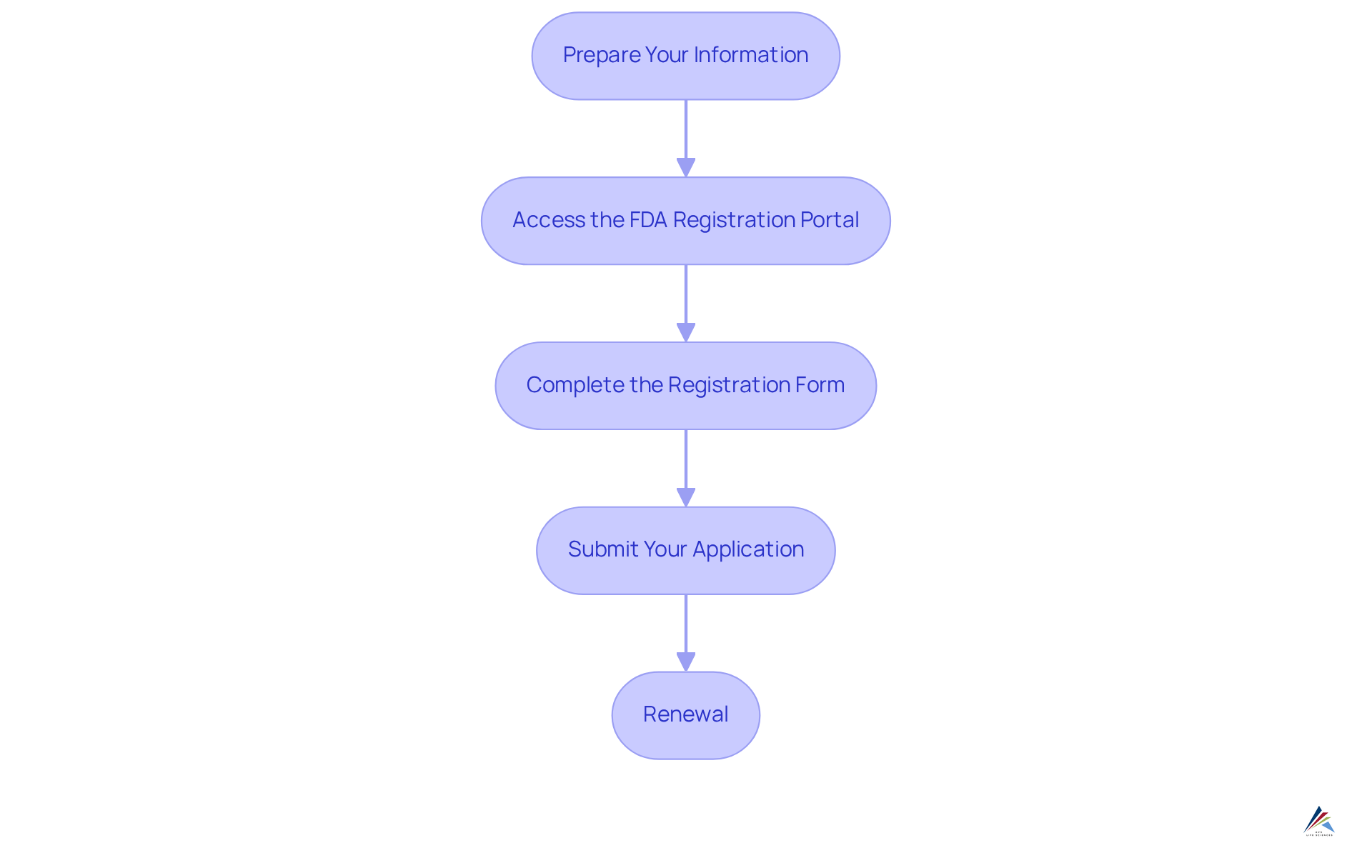
Follow the Step-by-Step Registration Process
To successfully register your cosmetic manufacturing facility, it is essential to follow these critical steps:
- Prepare Your Information: Gather all necessary details, including the establishment's name, address, and contact information for the responsible person.
- Access the FDA Registration Portal: Navigate to the FDA's Cosmetics Direct portal to initiate the application process. Ensure you possess a valid email address for communication purposes.
- Complete the Registration Form: Accurately fill out Form FDA 5066, providing comprehensive details about your facility and the products manufactured. Carefully review the information to avoid any errors.
- Submit Your Application: Once the form is complete, submit your application through the portal. A confirmation email will be sent once your application is processed.
- Renewal: Remember that . Set reminders to ensure compliance with this requirement.
By adhering to these steps, manufacturers can effectively navigate the facility registration for cosmetic and skincare manufacturers approval process and ensure compliance with regulatory standards.
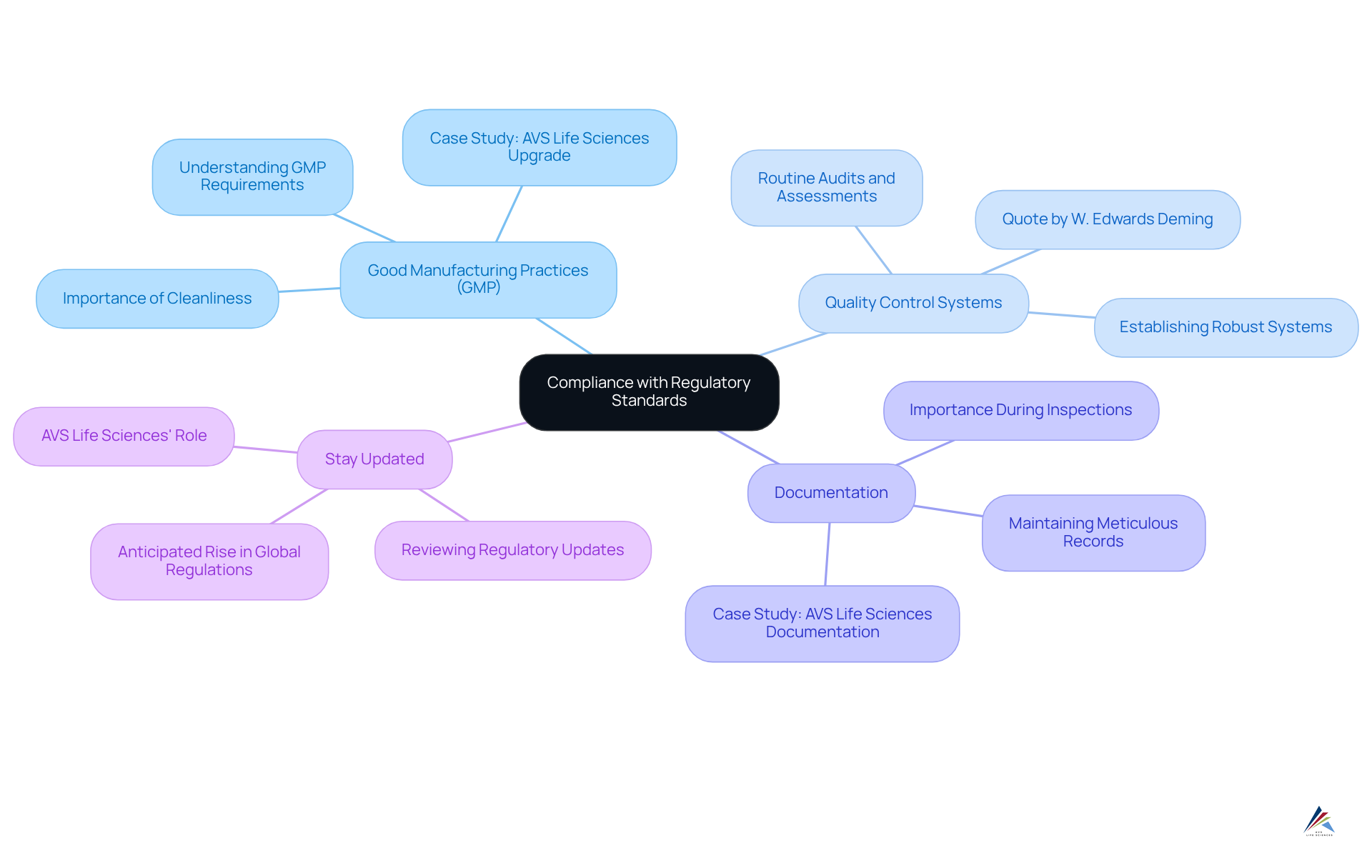
Ensure Compliance with Regulatory Standards
Compliance with regulatory standards extends beyond mere registration; it demands a steadfast commitment to ongoing adherence to various guidelines. Key compliance areas to focus on include:
- Good Manufacturing Practices (GMP): It is crucial to understand the specific GMP requirements for cosmetic manufacturing, which encompass maintaining cleanliness, ensuring proper documentation, and providing comprehensive employee training. A recent case involving AVS Life Sciences illustrates this point: they assisted a leading biotechnology company in upgrading their manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. This upgrade not only underscores the importance of adhering to stringent but also highlights the successful outcomes achieved through such enhancements.
- Quality Control Systems: Establishing robust quality control systems is essential for the continuous monitoring of product safety and efficacy. Routine audits and assessments are vital elements of an effective regulatory strategy, as they help identify areas for enhancement and guarantee adherence to standards. As W. Edwards Deming aptly stated, "uncontrolled variation is the enemy of quality," emphasizing the need for consistent quality management. AVS Life Sciences' experience in assisting clients with gap analysis and equipment installation further demonstrates their dedication to improving quality control, resulting in better adherence to standards.
- Documentation: Maintaining meticulous records of all manufacturing processes, quality checks, and employee training sessions is essential. This documentation is crucial during inspections and serves as proof of adherence efforts. In a recent case study, AVS Life Sciences' meticulous documentation practices were deemed suitable by the client's quality assurance team, reinforcing the importance of keeping detailed records that aid in successful compliance.
- Stay Updated: It is imperative to continuously review updates from the FDA and other regulatory bodies to ensure that your facility remains compliant with new regulations or changes to existing laws. With the anticipated rise in global regulations in 2025, manufacturers must prioritize adherence to navigate the evolving landscape effectively. AVS Life Sciences emphasizes the significance of remaining updated on regulatory changes to assist their clients in upholding standards.
By prioritizing these regulatory measures, manufacturers can foster trust with consumers and oversight agencies, ultimately enhancing their reputation in the competitive cosmetic market. Furthermore, leveraging the expertise of service providers like AVS Life Sciences, particularly following their recent acquisition of ValidPath, can significantly strengthen compliance efforts through tailored quality engineering solutions.
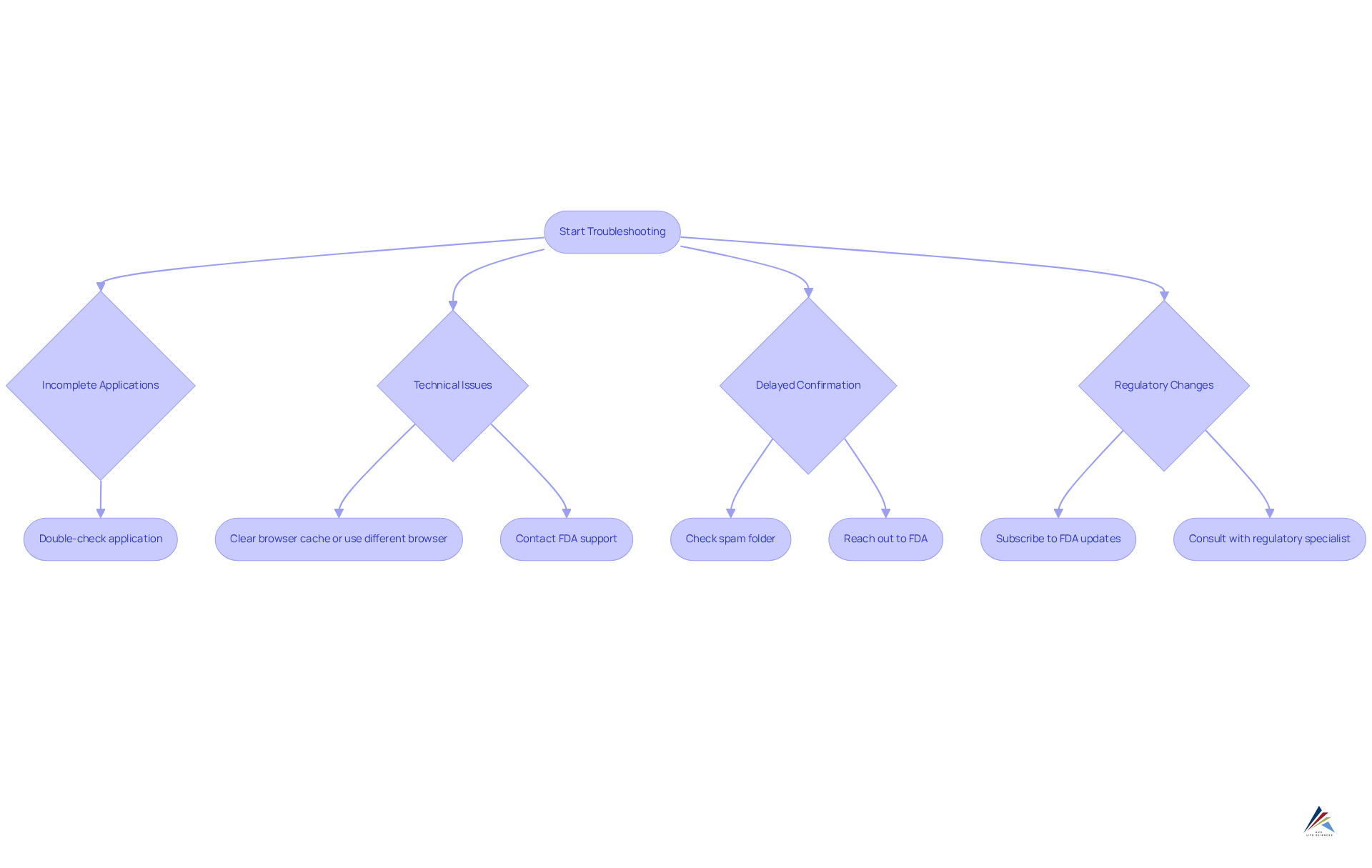
Troubleshoot Common Registration Challenges
Even with meticulous preparation, manufacturers may encounter challenges during the approval process. Understanding these common issues and their solutions is essential for ensuring a smooth experience:
- Incomplete Applications: It is crucial to ensure that all sections of the registration form are filled out completely. Double-check for any missing information prior to submission.
- Technical Issues: Should you experience technical difficulties with the FDA portal, consider clearing your browser cache or utilizing a different browser. If problems persist, do not hesitate to contact FDA support for assistance.
- Delayed Confirmation: If a confirmation email is not received within a few days, make sure to check your spam folder. If it is not found there, reach out to the FDA for further assistance.
- Regulatory Changes: Staying informed about any regulatory changes that may impact your enrollment is vital. Consider subscribing to FDA updates or consulting with a regulatory specialist as needed.
By proactively addressing these challenges, manufacturers can ensure a more seamless enrollment experience for facility registration for cosmetic and skincare manufacturers. Furthermore, leveraging AVS Life Sciences' will provide invaluable insights and support throughout the facility registration for cosmetic and skincare manufacturers process.
Conclusion
Understanding the process of facility registration for cosmetic and skincare manufacturers is essential for compliance with regulatory standards. This guide outlines the necessary steps and considerations to ensure that manufacturers not only meet the registration requirements but also uphold the quality and safety of their products. By grasping the nuances of the registration process, businesses can enhance their market readiness and compliance posture.
Key points discussed include:
- The importance of identifying the Responsible Person
- Understanding the necessity of registration under FDA guidelines
- Maintaining proper documentation
- The significance of adhering to Good Manufacturing Practices (GMP)
- Staying updated on regulatory changes, particularly those stemming from the Modernization of Cosmetics Regulation Act (MoCRA)
- Troubleshooting common registration challenges is crucial for a smooth application process.
In conclusion, navigating the complexities of facility registration is vital for cosmetic manufacturers aiming for success in a competitive market. By prioritizing compliance and leveraging expert guidance, such as that provided by AVS Life Sciences, manufacturers can not only meet regulatory standards but also build consumer trust and enhance their brand reputation. Taking proactive steps today will pave the way for a compliant and thriving business tomorrow.
Frequently Asked Questions
What is the role of the Responsible Person in cosmetic and skincare manufacturing?
The Responsible Person can be the manufacturer, packer, or distributor of cosmetic products. This entity is accountable for ensuring compliance with FDA regulations and must maintain records that substantiate the safety of the products.
Is facility registration necessary for all cosmetic manufacturers?
Yes, under the Modernization of Cosmetics Regulation Act (MoCRA), all cosmetic establishments are required to complete facility registration and follow Good Manufacturing Practices (GMP).
What documentation is required for facility registration?
Essential documentation includes proof of business establishment, facility address, and contact information. This documentation must be precise, thorough, and easily accessible for FDA inspections.
How can manufacturers stay updated on regulatory changes?
Manufacturers should stay informed about any changes in regulations, especially those introduced by MoCRA, which affect compliance requirements.
What is the new labeling requirement introduced by MoCRA?
As of December 29, 2024, the labeling requirement for the Responsible Person will become mandatory, necessitating that their name, domestic address, and phone number be included on product packaging.
How can manufacturers ensure a smooth facility registration process?
By fully understanding the registration requirements and staying informed about regulatory updates, manufacturers can avoid common obstacles and promote a smoother facility registration process. Collaborating with experts, such as AVS Life Sciences, can also provide valuable advice and solutions in quality management and regulatory compliance.
