4 Steps for Cosmetic Labeling Compliance for Emerging Brands

Overview
The article centers on the essential steps that emerging brands must undertake to achieve compliance with cosmetic labeling regulations. This involves not only a thorough understanding of the regulations but also the creation of accurate labels and the maintenance of comprehensive documentation. Key regulatory components are outlined, including:
- Ingredient declarations
- Identity statements
- Necessary warnings
Furthermore, the article underscores the critical need for brands to stay informed about compliance changes to avert potential enforcement issues and to bolster consumer trust. By engaging with these compliance solutions, brands can not only meet regulatory requirements but also enhance their market reputation.
Introduction
Navigating the complex landscape of cosmetic labeling regulations presents a significant challenge for emerging brands. As the demand for transparency in the cosmetics industry intensifies, comprehending the intricacies of compliance becomes not merely advantageous—it is essential for cultivating consumer trust and circumventing costly penalties.
As brands endeavor to align with the stringent standards established by authorities such as Health Canada and the FDA, a critical question emerges: how can they ensure that their labels fulfill all necessary requirements while simultaneously distinguishing themselves in a competitive marketplace?
This guide delves into the pivotal steps and best practices for achieving cosmetic labeling compliance, empowering brands to excel in an ever-evolving regulatory landscape.
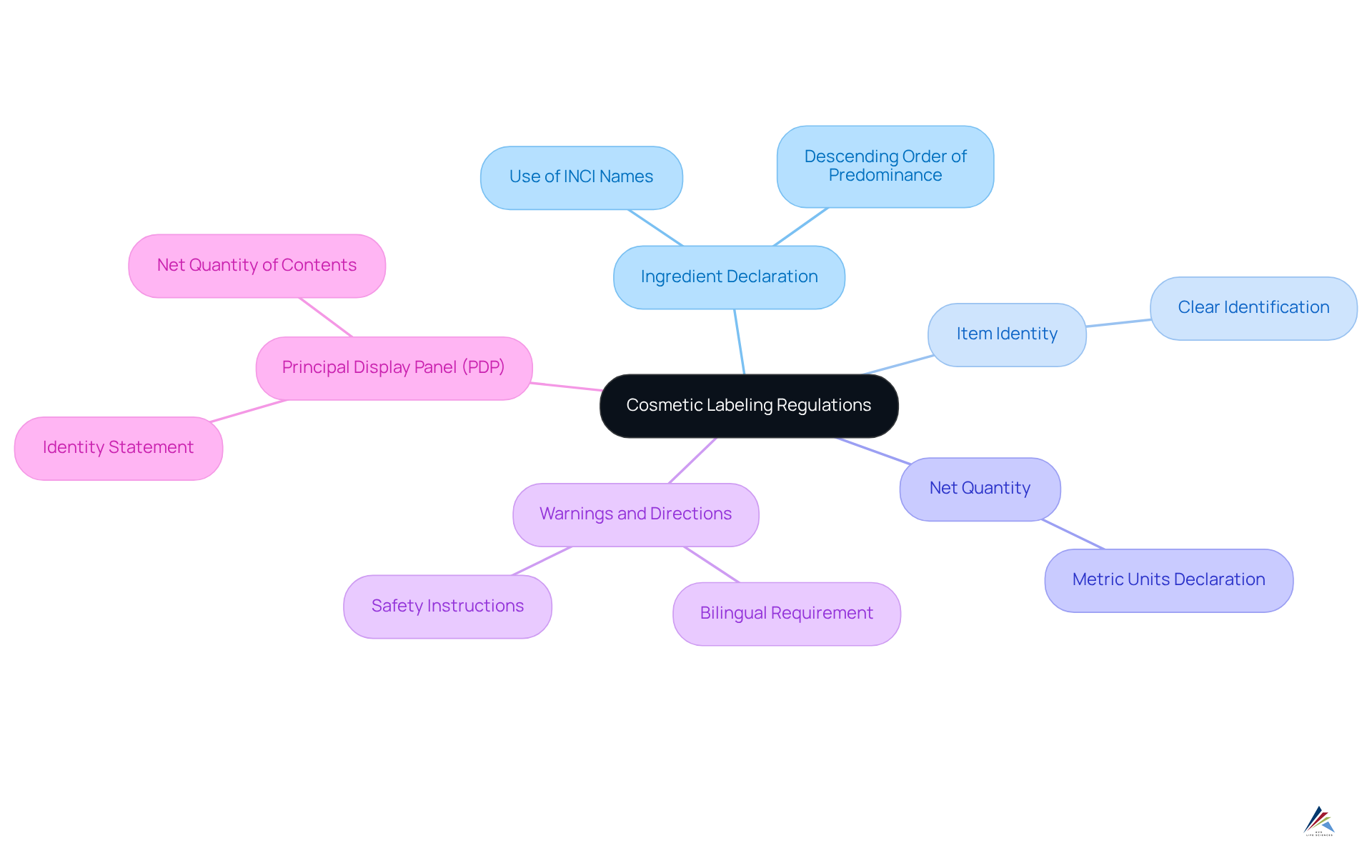
Understand Cosmetic Labeling Regulations
To achieve cosmetic labeling compliance for emerging brands, it is essential to understand the specific regulations set forth by Health Canada and other governing bodies. Key components of these regulations include:
- Ingredient Declaration: All ingredients must be listed in descending order of predominance, utilizing the International Nomenclature of Cosmetic Ingredients (INCI) names. This ensures consistency and clarity for consumers, allowing them to identify allergens and make informed choices.
- Item Identity: The label needs to clearly indicate the item's identity, offering consumers prompt recognition of what they are buying.
- Net Quantity: It is crucial to indicate the net quantity of the item in metric units, ensuring transparency regarding the amount contained within the packaging.
- Warnings and Directions: Any necessary warnings or usage instructions are required to inform consumers about safe usage practices, particularly for products that may pose risks, such as hair dyes or products containing specific allergens.
- Principal Display Panel (PDP): The PDP must include an Identity Statement and Net Quantity of Contents, which are critical for consumer recognition and compliance.
Recent updates to Health Canada's marking requirements emphasize the necessity for complete contact details on internal tags, effective from April 2024. This change reflects a growing demand for transparency in the cosmetics industry, with 62% of Canadian consumers actively using skin-care products, highlighting the importance of clear labeling. Additionally, labels must include information in both French and English to comply with Canada's bilingual nature.
Successful examples from emerging brands demonstrate the effectiveness of adhering to cosmetic labeling compliance for emerging brands. Brands that prioritize cosmetic labeling compliance for emerging brands not only enhance consumer trust but also reduce the risk of regulatory issues. As Estella Benz points out, 'The demand for transparency in the cosmetics industry has grown significantly in recent years,' underscoring the importance of precise identification practices. Moreover, it is significant to mention that there is presently no obligation to include an expiration date on Canadian cosmetic items, which can affect adherence strategies.
For additional assistance, consult Health Canada's Industry Guide, which offers extensive information on labeling requirements and best practices for adherence.
Follow Step-by-Step Compliance Procedures
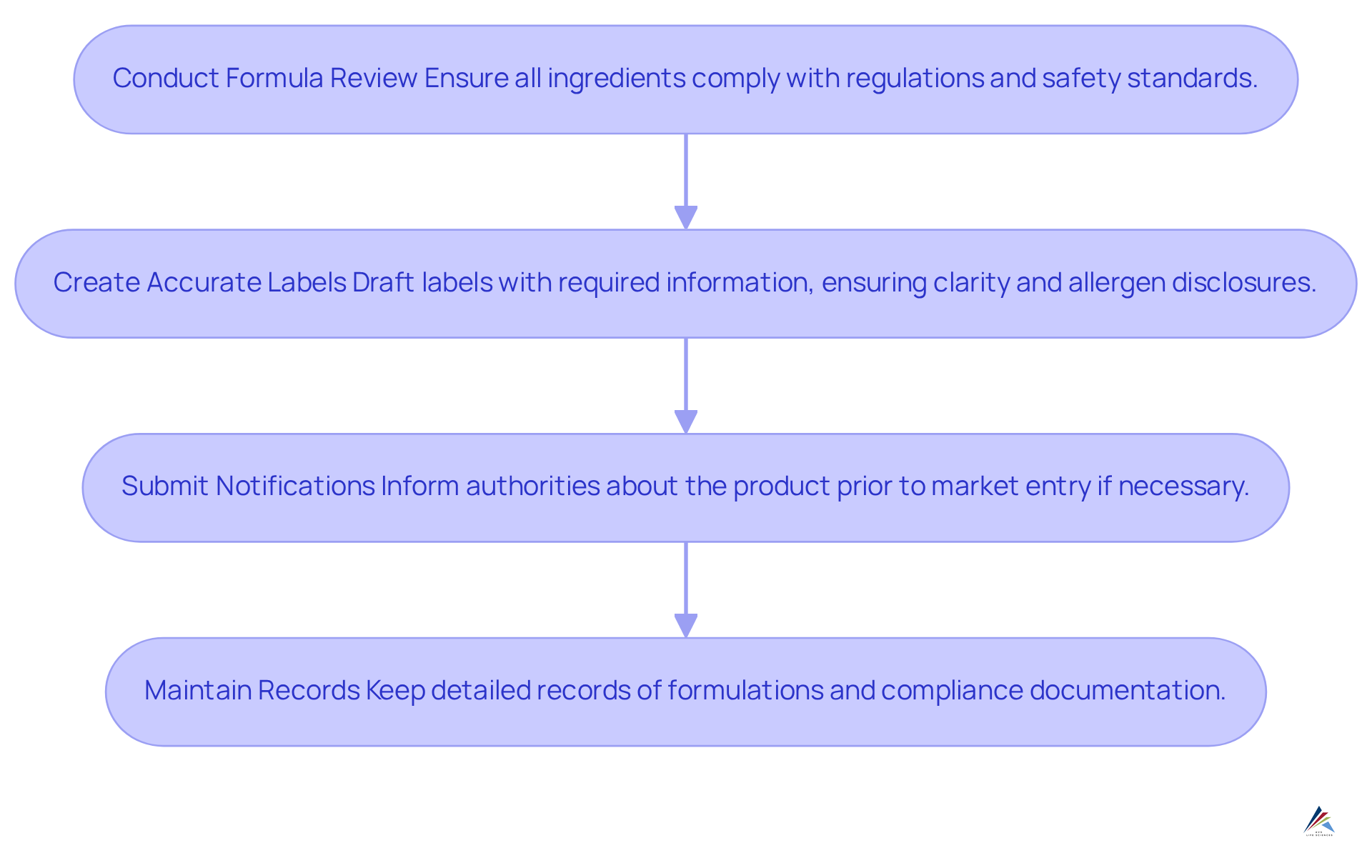
To achieve compliance, it is essential to follow these steps:
-
Conduct a Formula Review: Begin by ensuring that all ingredients are compliant with regulations and safe for use. This involves verifying that all components meet the safety substantiation standards established by the Modernization of Cosmetics Regulation Act (MoCRA).
-
Create Accurate Labels: Draft labels that encompass all required information, ensuring clarity and legibility. Labels must comply with MoCRA guidelines, which stipulate that font sizes are easily readable, even on smaller containers, and that allergen disclosures are prominently displayed. Moreover, all cosmetic items must clearly show responsible individual contact details by December 29, 2024.
-
Submit Notifications: If necessary, inform the appropriate authorities (e.g., Health Canada) about your item prior to its market entry. This step is crucial for upholding regulations and preventing potential enforcement measures, such as item confiscations or penalties.
-
Maintain Records: Keep detailed records of all formulations, markings, and compliance documentation to facilitate audits and inspections. Ingredient lists for marketed items must be supplied and revised annually, which is vital for demonstrating compliance with Good Manufacturing Practices (GMP) and addressing any inquiries from regulatory authorities.
Utilize checklists from resources like the MoCRA Cosmetic Label Requirements to ensure nothing is overlooked. Statistics indicate that cosmetic labeling compliance for emerging brands is vital, as non-compliance can lead to significant repercussions, including enforcement actions and damage to brand reputation, making thorough preparation and adherence to these steps essential for success. Furthermore, ensure that fragrance ingredients are listed in a clear format, ideally grouped together, with allergens highlighted, and consider using contrasting backgrounds on labels to enhance text visibility.
Implement Effective Documentation Practices
Effective documentation practices are crucial for ensuring cosmetic labeling compliance for emerging brands and for maintaining product integrity. To achieve this, consider the following strategies:
- Maintain a Product Information File (PIF): This file should encompass all relevant details about the product, including formulations, safety assessments, and labeling.
- Document Changes: It is essential to keep meticulous records of any modifications made to formulations or descriptions, along with the rationale for those changes.
- Audit Trails: Establish a robust system for tracking all compliance-related documents, ensuring they are readily accessible for audits.
- Training Records: Document all training sessions for staff on regulatory procedures and labeling requirements.
For best practices, refer to resources like the FDA's Good Manufacturing Practice Guidelines. By implementing these documentation practices, organizations can enhance their compliance efforts, particularly in terms of cosmetic labeling compliance for emerging brands, and ensure a high standard of product quality.

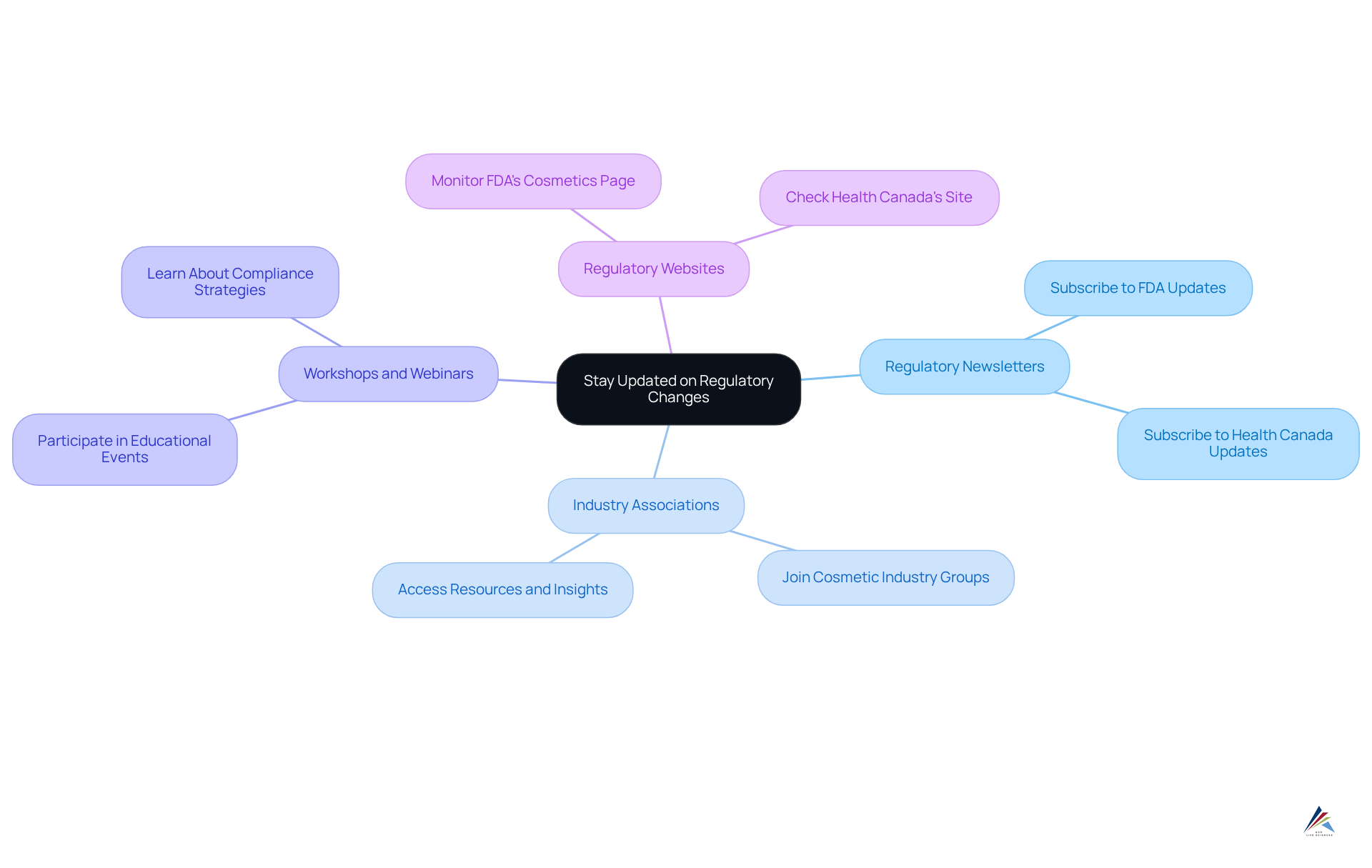
Stay Updated on Regulatory Changes
To stay updated on compliance challenges in the cosmetic industry:
- Subscribe to Regulatory Newsletters: Sign up for updates from organizations such as the FDA and Health Canada to receive timely information.
- Join Industry Associations: Engage with groups dedicated to cosmetic regulations for valuable resources and insights.
- Attend Workshops and Webinars: Participate in educational events that address recent regulatory changes and compliance strategies.
- Monitor Regulatory Websites: Regularly check the FDA's Cosmetics page and Health Canada's site for the latest updates.
By actively engaging with these resources, brands can ensure their cosmetic labeling compliance for emerging brands with the latest regulations, thereby reinforcing their commitment to industry standards.
Conclusion
Understanding and adhering to cosmetic labeling compliance is essential for emerging brands aiming to establish themselves in a competitive market. By effectively navigating the complex regulations set forth by authorities like Health Canada, brands can significantly enhance consumer trust and mitigate the risk of regulatory issues. The foundational components of compliance—including ingredient declaration, item identity, net quantity, and appropriate warnings—are crucial for effective labeling practices that resonate with informed consumers.
This article outlines a systematic approach to achieving compliance, highlighting the importance of:
- Conducting thorough formula reviews
- Creating precise labels
- Submitting necessary notifications
- Maintaining meticulous records
Effective documentation practices not only facilitate compliance but also ensure product integrity, which is vital for building a reputable brand. Staying informed about regulatory changes through newsletters, industry associations, and workshops empowers brands to adapt to evolving standards and maintain compliance seamlessly.
Ultimately, prioritizing cosmetic labeling compliance transcends mere legal requirements; it fosters transparency and accountability within the cosmetics industry. Emerging brands that adopt these practices position themselves as trustworthy players in the market, poised to meet consumer expectations and adeptly navigate the complexities of regulation. By taking proactive steps and leveraging available resources, brands can ensure their products are compliant and well-received, thereby paving the way for long-term success.
Frequently Asked Questions
What are the key components of cosmetic labeling regulations in Canada?
Key components include ingredient declaration, item identity, net quantity, warnings and directions, and the principal display panel (PDP) requirements.
How should ingredients be listed on cosmetic labels?
Ingredients must be listed in descending order of predominance using the International Nomenclature of Cosmetic Ingredients (INCI) names.
What does the item identity on a cosmetic label refer to?
The item identity refers to a clear indication of what the product is, allowing consumers to recognize it promptly.
What is required regarding the net quantity on cosmetic labels?
The net quantity must be indicated in metric units to ensure transparency about the amount contained within the packaging.
Why are warnings and directions necessary on cosmetic labels?
Warnings and directions are necessary to inform consumers about safe usage practices, especially for products that may pose risks.
What must the principal display panel (PDP) include?
The PDP must include an Identity Statement and the Net Quantity of Contents for consumer recognition and compliance.
What recent updates have been made to Health Canada's marking requirements?
As of April 2024, complete contact details must be included on internal tags to enhance transparency in the cosmetics industry.
What languages must the labels be in to comply with Canadian regulations?
Labels must include information in both French and English to comply with Canada's bilingual requirements.
What is the significance of cosmetic labeling compliance for emerging brands?
Compliance enhances consumer trust and reduces the risk of regulatory issues, which is crucial for emerging brands.
Is there an obligation to include expiration dates on Canadian cosmetic items?
Currently, there is no obligation to include expiration dates on Canadian cosmetic items.
Where can brands find additional information on labeling requirements?
Brands can consult Health Canada's Industry Guide for extensive information on labeling requirements and best practices.
