4 Key Strategies for USP 661 Compliance Success

Overview
The four key strategies for achieving USP 661 compliance success are:
- Material characterization
- Rigorous testing protocols
- Comprehensive risk assessments
- Implementation of best practices, including training and documentation
These strategies are essential for ensuring product safety and efficacy, as they enable organizations to align their operations with regulatory expectations. By adopting structured approaches and committing to continuous improvement, organizations can significantly enhance overall product quality. Embracing these strategies not only addresses compliance challenges but also empowers companies to thrive in a competitive market.
Introduction
Navigating the complexities of pharmaceutical regulations presents a formidable challenge, particularly with the introduction of stringent standards such as USP 661. This framework establishes essential criteria for evaluating the safety and efficacy of materials utilized in drug production, transforming compliance from a mere regulatory hurdle into a pathway for enhanced product quality. As organizations endeavor to align with these standards, the real challenge lies in the effective implementation of best practices that not only meet but exceed compliance expectations.
What strategies can be employed to ensure success in achieving USP 661 compliance while fostering a culture of continuous improvement? By exploring proven methodologies and insights, organizations can not only comply but thrive in this evolving landscape.
Understand USP 661: Key Principles and Requirements
USP 661 delineates the essential criteria for evaluating the physical and chemical characteristics of substances that are utilized in pharmaceutical production. The key principles encompass:
- Material Characterization: A thorough understanding of materials' properties, including their chemical composition and physical characteristics, is paramount for ensuring product safety and efficacy. Recent statistics reveal that effective substance characterization can drastically reduce the incidence of out-of-specification results, with a robust correlation of 0.96 between the right-first-time rate and total deviations, thereby enhancing overall product quality.
- Testing Protocols: The establishment of rigorous testing protocols to evaluate substances against USP standards is vital for compliance. This includes both in-process and final product testing, ensuring that all materials satisfy stringent quality criteria. Industry leaders, such as Steven Walfish, emphasize that stringent testing protocols not only facilitate adherence to standards but also foster confidence in product integrity. Walfish asserts, "An ongoing program to collect and analyze product and process data that relate to product quality must be established."
- Risk Assessment: Conducting comprehensive risk evaluations to pinpoint potential regulatory gaps and areas for enhancement is critical for maintaining compliance with USP 661 regulations. By proactively addressing these gaps, organizations can bolster their regulatory posture and mitigate risks associated with product quality. Implementing structured resolution strategies can yield higher success rates in characterization activities.
Familiarizing themselves with these principles empowers organizations to elevate their readiness for regulatory initiatives, aligning operations with regulatory expectations and ultimately ensuring the safety and efficacy of pharmaceutical products.

Implement Best Practices for USP 661 Compliance
To achieve , organizations must adopt best practices that ensure effective implementation and adherence to the standard.
- Conduct Regular Training: It is essential that all staff involved in material handling and testing receive thorough training on USP 661 requirements and best practices. This foundational step not only enhances individual competence but also fortifies the organization’s overall compliance posture in accordance with USP 661.
- Develop Standard Operating Procedures (SOPs): Organizations should create detailed SOPs that outline the processes for material evaluation, testing, and documentation. This structured approach guarantees consistency and adherence to regulatory standards, ultimately fostering a culture of compliance.
- Utilize Technology: Implementing software solutions that facilitate real-time monitoring of regulatory activities is crucial. By automating documentation processes, organizations significantly reduce the likelihood of human error, ensuring that compliance is not only achievable but sustainable.
- Engage in Continuous Improvement: Organizations must regularly review and update their adherence methods. By incorporating feedback, audit findings, and changes in regulatory requirements, they can maintain alignment with USP 661, which demonstrates their commitment to excellence and adaptability in a dynamic regulatory landscape.

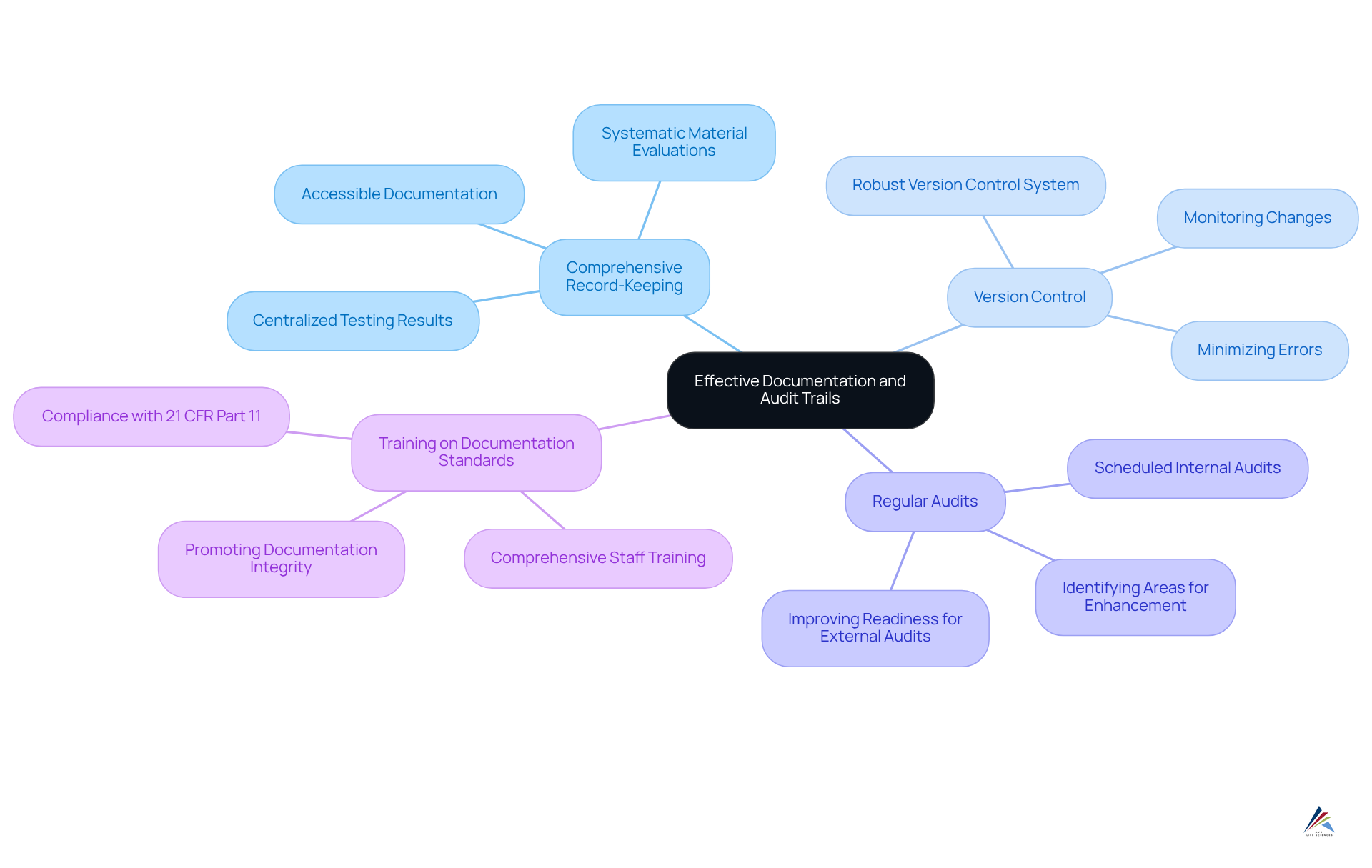
Maintain Effective Documentation and Audit Trails
Maintaining effective documentation and audit trails is essential for compliance with USP 661 and involves several best practices:
- Comprehensive Record-Keeping: Centralize all testing results, material evaluations, and compliance activities in a systematic manner. This approach ensures that documentation is easily accessible and retrievable during audits, facilitating a smoother review process.
- Version Control: Implement a robust version control system for all documents. This method allows organizations to monitor changes meticulously, guaranteeing that the latest procedures are consistently adhered to, thereby minimizing the risk of errors.
- Regular Audits: Schedule internal audits at predefined intervals to assess adherence to documentation practices. This proactive method not only identifies areas for enhancement but also occur, thereby improving overall readiness. Audit trails are vital for both internal and external audits, ensuring transparency and accountability in regulated industries.
- Training on Documentation Standards: Provide comprehensive training for staff regarding the significance of accurate documentation and the specific requirements outlined in USP 661. Promoting a culture of adherence through education ensures that all personnel understand their roles in preserving documentation integrity. Furthermore, it is essential that electronic signatures comply with 21 CFR Part 11, underscoring the importance of adhering to regulations in the context of electronic records. Effective documentation methods pertain to nearly all delivery areas and nations, highlighting their global significance. Moreover, adherence to Good Manufacturing Practices (GMP) and ISO standards is crucial for upholding regulations in the pharmaceutical industry.
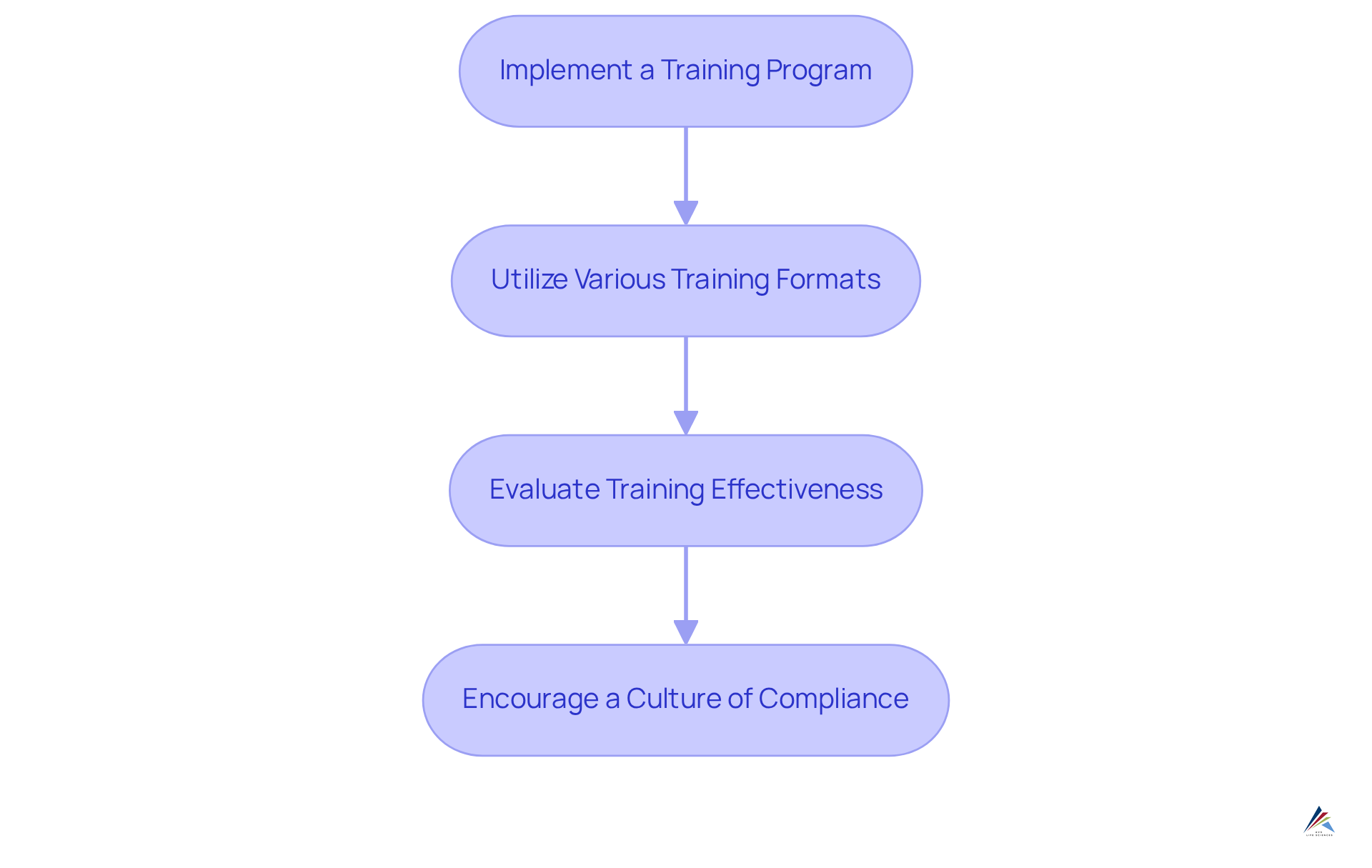
Provide Continuous Training on Compliance Standards
To ensure ongoing compliance with USP 661, organizations must take decisive action:
- Implement a Training Program: Develop a structured training program that comprehensively covers USP 661 requirements, compliance best practices, and updates on regulatory changes.
- Utilize Various Training Formats: Incorporate a diverse array of training formats, including workshops, e-learning modules, and hands-on sessions, to address different learning styles and enhance knowledge retention.
- Evaluate Training Effectiveness: Regularly assess the effectiveness of training programs through feedback surveys and knowledge assessments, identifying areas for improvement to ensure continuous enhancement.
- Encourage a Culture of Compliance: Foster an organizational culture that prioritizes compliance by recognizing and rewarding employees who demonstrate a steadfast commitment to upholding standards.
Conclusion
Achieving compliance with USP 661 is essential for organizations in the pharmaceutical industry, as it ensures the safety and efficacy of products through stringent standards. By understanding key principles such as material characterization, rigorous testing protocols, and effective risk assessments, organizations can align their operations with regulatory expectations and enhance product quality.
The article highlights several critical strategies for ensuring compliance, including:
- The implementation of comprehensive training programs
- The development of detailed standard operating procedures
- The utilization of technology for real-time monitoring
Additionally, maintaining effective documentation and audit trails is crucial for transparency and accountability. Regular audits and continuous improvement practices further solidify an organization’s commitment to upholding USP 661 standards.
Ultimately, the significance of USP 661 compliance cannot be overstated. Organizations are encouraged to prioritize these strategies not only to meet regulatory requirements but also to foster a culture of excellence and accountability. By embracing these best practices, companies can navigate the complexities of compliance, ensuring their products meet the highest standards of safety and quality in the pharmaceutical landscape.
Frequently Asked Questions
What is USP 661 and what does it cover?
USP 661 delineates the essential criteria for evaluating the physical and chemical characteristics of substances used in pharmaceutical production, ensuring product safety and efficacy.
Why is material characterization important in pharmaceutical production?
Material characterization is crucial because it provides a thorough understanding of materials' properties, including their chemical composition and physical characteristics, which can significantly reduce the incidence of out-of-specification results and enhance overall product quality.
What is the correlation between effective substance characterization and product quality?
There is a robust correlation of 0.96 between the right-first-time rate and total deviations, indicating that effective substance characterization can drastically improve product quality.
What are the key components of testing protocols under USP 661?
Key components include establishing rigorous testing protocols for both in-process and final product testing to ensure that all materials meet stringent quality criteria.
How do stringent testing protocols benefit pharmaceutical companies?
Stringent testing protocols facilitate adherence to USP standards and foster confidence in product integrity, as emphasized by industry leaders like Steven Walfish.
What role does risk assessment play in compliance with USP 661?
Conducting comprehensive risk evaluations helps identify potential regulatory gaps and areas for enhancement, which is critical for maintaining compliance with USP 661 regulations.
How can organizations improve their regulatory posture in relation to USP 661?
Organizations can improve their regulatory posture by proactively addressing regulatory gaps and implementing structured resolution strategies, which can lead to higher success rates in characterization activities.
What is the overall benefit of familiarizing oneself with the principles of USP 661?
Familiarizing with these principles empowers organizations to elevate their readiness for regulatory initiatives, align operations with regulatory expectations, and ensure the safety and efficacy of pharmaceutical products.
