4 Key Strategies for Quality Compliance in Pharmaceuticals

Overview
The four key strategies for quality compliance in pharmaceuticals are crucial for addressing the inherent challenges in the industry. These strategies encompass:
- Understanding regulatory frameworks
- Establishing robust documentation and audit trails
- Implementing continuous training and education programs
- Cultivating a culture of compliance and quality
By emphasizing these strategies, pharmaceutical companies can ensure adherence to regulations, enhance operational efficiency, and foster a knowledgeable workforce. This approach not only empowers organizations to navigate the complexities of quality compliance effectively but also positions them for sustained success in a highly regulated environment.
Introduction
Navigating the complex landscape of pharmaceutical regulations is undeniably daunting; however, it is crucial for ensuring product quality and safety. With regulatory bodies applying increasing scrutiny and compliance standards continually evolving, pharmaceutical companies encounter both significant challenges and promising opportunities in their pursuit of quality assurance. This article explores four key strategies that organizations can implement to enhance their quality compliance efforts, ultimately fostering a culture of excellence.
How can companies not only meet but exceed these regulatory demands to safeguard their reputation and ensure patient safety?
Understand Regulatory Frameworks and Standards
Pharmaceutical companies must thoroughly understand the various regulatory frameworks governing their operations. Key regulations, such as Good Manufacturing Practices (GMP), are critical as they ensure quality compliance by consistently producing and controlling products according to established standards. Additionally, ISO standards provide essential guidelines for quality management systems. Understanding is equally crucial for quality compliance in the U.S. market.
To maintain quality compliance, businesses should:
- Regularly assess updates from oversight organizations like the FDA and EMA, staying informed of changes that could impact their compliance status.
- Engage with industry groups to gain valuable insights into best practices and emerging developments in regulatory adherence.
Establish Robust Documentation and Audit Trails
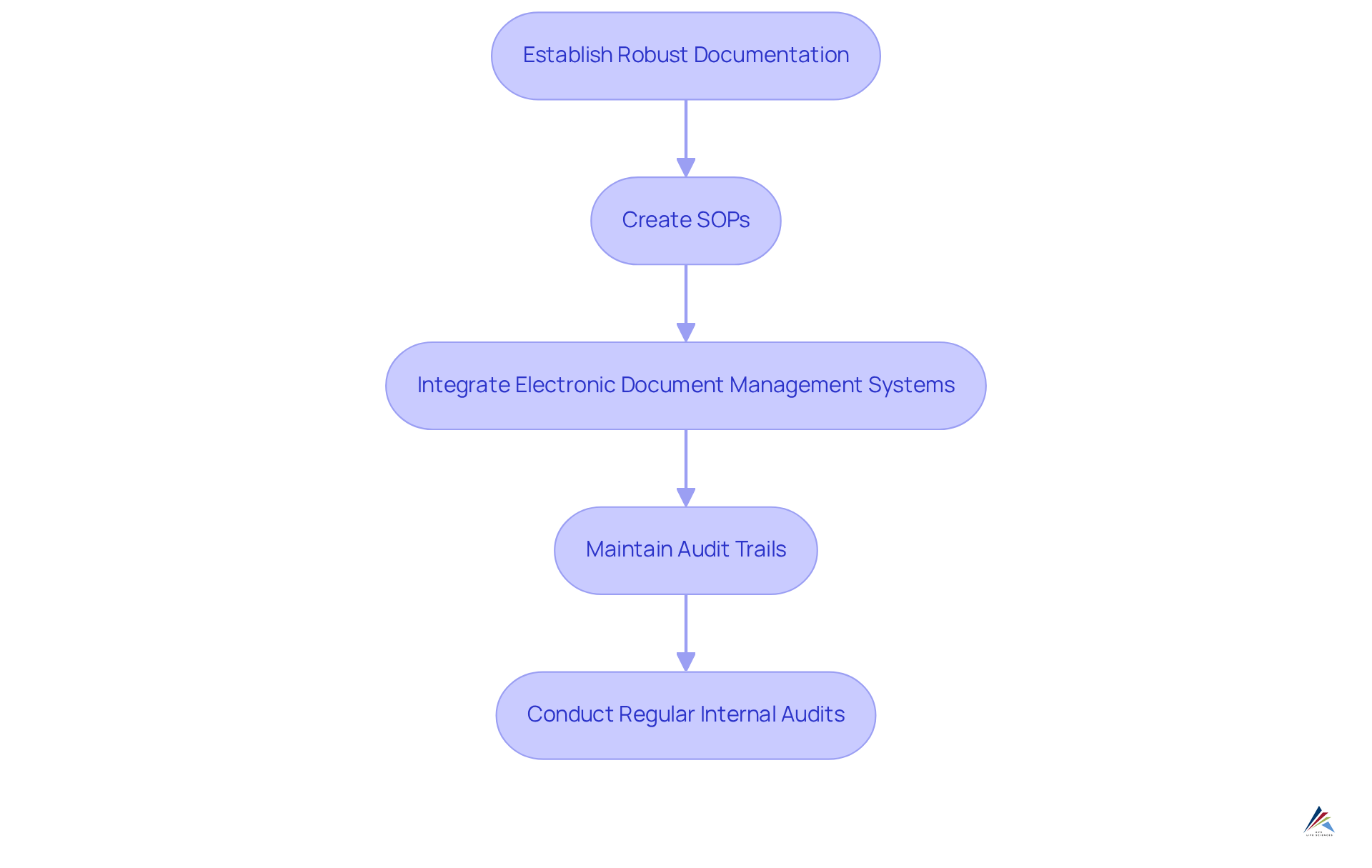
To attain conformity, pharmaceutical firms must adopt thorough that guarantee precise records of all processes, changes, and decisions. This begins with the creation of operating procedures (SOPs) that clearly outline processes in a concise manner. According to Stan W. Wollen, the developer of ALCOA principles, records should be 'Attributable, Legible, Contemporaneous, Original, and Accurate.'
The integration of electronic document management systems significantly enhances the traceability and accessibility of these documents, facilitating compliance with regulatory standards. Furthermore, maintaining detailed audit trails is essential; these trails should meticulously capture all modifications made to documents, including the identity of the individual who made the change and the timestamp of the alteration.
Regular internal audits are crucial to confirm compliance with documentation standards and to identify areas for enhancement. Notably, 80% of data integrity-related warning letters were issued from 2014 to 2018, underscoring the essential requirement for robust documentation methods. This proactive approach not only assists in adhering to regulations but also enhances overall operational efficiency, enabling organizations to effectively manage the complexities of the pharmaceutical landscape.
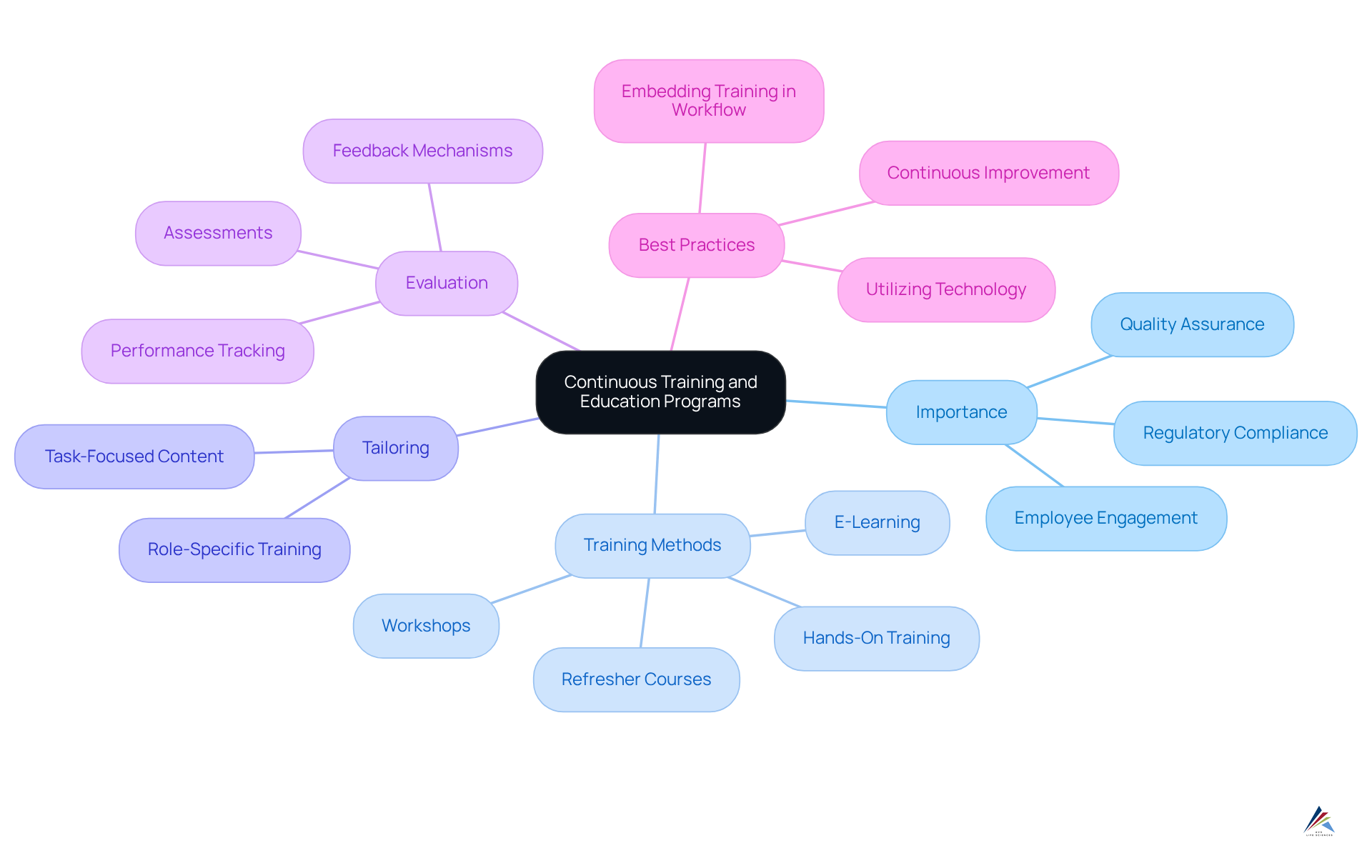
Implement Continuous Training and Education Programs
Introducing ongoing training and education initiatives is vital for ensuring that all employees are well-informed about current legal requirements and quality compliance methods. In the pharmaceutical industry, it is crucial that employees receive training at least annually to maintain quality compliance with evolving regulations. Training should be tailored to different roles within the organization, focusing on specific tasks and responsibilities related to . Regular workshops, e-learning modules, and refresher courses serve to reinforce knowledge and skills. Moreover, hands-on training experiences enable employees to absorb information more thoroughly and quickly. Nurturing a culture of learning motivates employees to remain engaged and proactive regarding regulations.
As William Foster aptly stated, "Quality is never an accident; it is always the result of high intention, sincere effort, intelligent direction, and skillful execution." Companies must evaluate the effectiveness of their training programs through assessments and feedback to ensure they meet the evolving needs of the industry. This includes addressing potential pitfalls, such as outdated content or lack of engagement, which can hinder the effectiveness of training initiatives.
AVS Life Sciences provides comprehensive training programs that exemplify these best practices, ensuring that organizations are well-equipped to navigate the complexities of quality compliance environments. By implementing these strategies, companies can foster a compliant workforce that is both knowledgeable and responsive to regulatory changes.
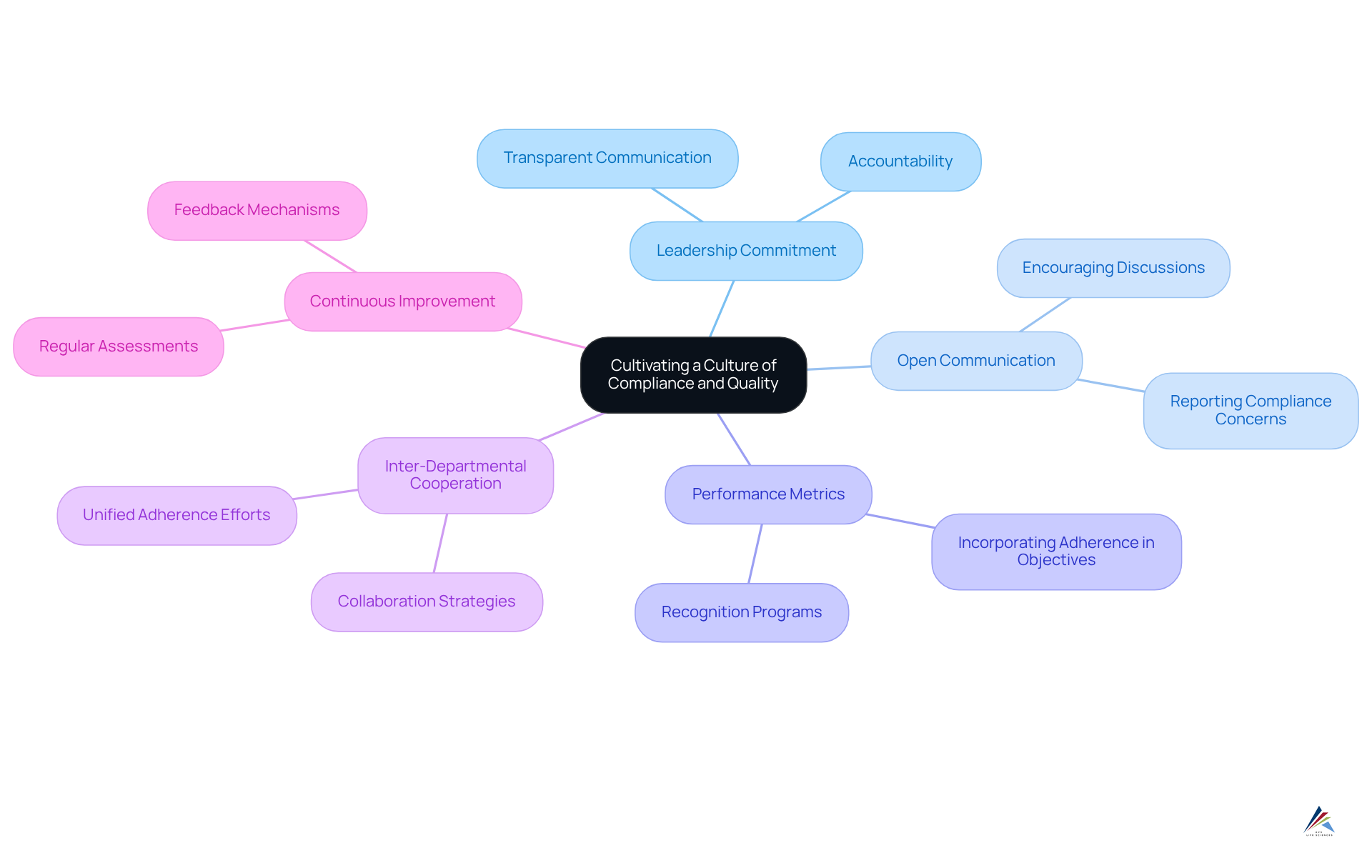
Cultivate a Culture of Compliance and Quality
Fostering a culture of adherence and quality within an organization is crucial for maintaining high standards and ensuring regulatory conformity, particularly in alignment with Good Manufacturing Practices (GMP), ISO standards, and Quality System Regulations (QSR).
Leadership must establish the tone by demonstrating a commitment to adherence through transparent communication and accountability. By promoting open discussions regarding adherence matters and recognizing staff who exemplify adherence behaviors, organizations can significantly strengthen this culture.
Moreover, incorporating adherence into performance metrics and organizational objectives ensures that all employees acknowledge its importance. Encouraging cooperation among departments is essential for unified adherence efforts.
Regular assessments of the organizational culture through surveys and feedback can identify areas for improvement and reinforce the , understanding that quality excellence is a continuous process necessitating long-term dedication.
Conclusion
In the pharmaceutical industry, ensuring quality compliance transcends mere regulatory obligation; it is a cornerstone of operational integrity and business success. By implementing strategic approaches—such as understanding regulatory frameworks, maintaining robust documentation, providing continuous training, and cultivating a culture of compliance—organizations can adeptly navigate the complexities of this highly regulated environment.
This article underscores four pivotal strategies essential for achieving quality compliance:
- A comprehensive understanding of regulations like GMP and ISO standards empowers companies to align their practices with industry expectations.
- Establishing meticulous documentation and audit trails guarantees that all processes remain transparent and traceable.
- Continuous training programs equip employees with the knowledge necessary to adapt to evolving regulations.
- Fostering a culture of compliance promotes a shared commitment to quality across all organizational levels.
The significance of quality compliance in pharmaceuticals is paramount. It not only safeguards public health but also enhances organizational reputation and operational efficiency. By prioritizing these strategies, pharmaceutical companies can construct a resilient framework that not only meets regulatory requirements but also propels excellence in product quality and safety. Embracing a proactive stance on compliance will yield sustainable growth and a robust commitment to the highest standards of quality in the industry.
Frequently Asked Questions
What is the significance of regulatory frameworks in the pharmaceutical industry?
Regulatory frameworks are essential for pharmaceutical companies as they govern operations and ensure quality compliance, helping to consistently produce and control products according to established standards.
What are Good Manufacturing Practices (GMP)?
Good Manufacturing Practices (GMP) are key regulations that ensure quality compliance by requiring pharmaceutical companies to consistently produce and control their products according to established standards.
Why are ISO standards important for pharmaceutical companies?
ISO standards provide essential guidelines for quality management systems, helping pharmaceutical companies maintain high levels of quality and compliance in their operations.
What are Quality System Regulations (QSR)?
Quality System Regulations (QSR) are crucial for ensuring quality compliance specifically in the U.S. market, outlining the necessary requirements for quality management in pharmaceutical manufacturing.
How can pharmaceutical companies maintain quality compliance?
Companies can maintain quality compliance by regularly assessing updates from oversight organizations like the FDA and EMA, and engaging with industry groups to gain insights into best practices and emerging developments in regulatory adherence.
