4 Key Steps to Choose a Regulatory Compliance Consultant

Overview
To effectively choose a regulatory compliance consultant, organizations must first assess their specific compliance needs. Evaluating potential consultants' industry expertise is crucial, as is establishing clear communication protocols for collaboration. Understanding regulatory goals and fostering open communication are essential for successful partnerships. This is exemplified by AVS Life Sciences' proven track record in enhancing compliance within the life sciences sector. By leveraging their expertise, organizations can navigate compliance challenges more effectively and achieve their regulatory objectives.
Introduction
Navigating the intricate web of regulations in the life sciences sector presents a significant challenge, as organizations encounter increasing pressures to comply with standards such as Good Manufacturing Practices and ISO regulations. The right regulatory compliance consultant can effectively mitigate the risks associated with non-compliance—risks that can amount to millions—while simultaneously enhancing operational efficiency and product integrity. Yet, with a plethora of options available, how can organizations ensure they select a consultant who genuinely aligns with their specific needs and challenges? This article explores the essential steps for choosing a regulatory compliance consultant, offering insights that can lead to informed decisions and fruitful partnerships.
Understand Regulatory Compliance Consulting in Life Sciences
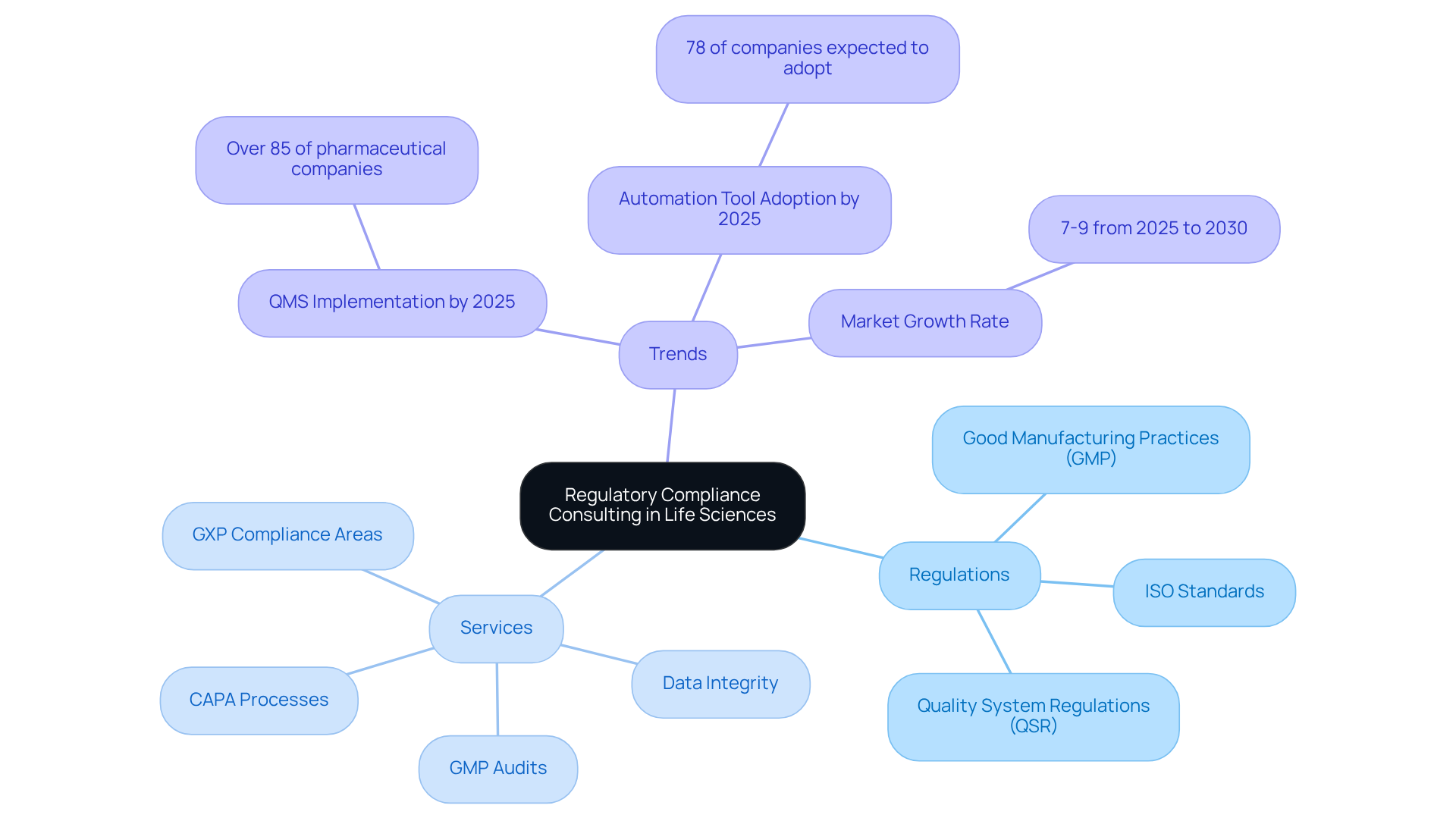
A regulatory compliance consultant in life sciences is essential for organizations seeking to navigate the complex regulations that govern the industry, including:
AVS Life Sciences offers comprehensive GXP compliance services, focusing on critical areas such as:
- GMP audits for APIs
- Drug products
- Testing facilities
- Data integrity
- CAPA processes
These regulatory compliance consultants offer tailored approaches that not only ensure compliance but also enhance operational efficiency. As the regulatory landscape evolves, understanding these frameworks becomes vital for maintaining product integrity, safeguarding patient safety, and mitigating the financial risks associated with non-compliance, which can average over 5 million USD for organizations facing significant compliance issues.
A skilled regulatory compliance consultant assists in establishing robust regulatory frameworks aligned with industry best practices, fostering a culture of quality and accountability. With over 85% of pharmaceutical companies projected to implement by 2025, the role of a regulatory compliance consultant for effective consulting on adherence to standards is increasingly critical.
Recent trends indicate that 78% of organizations are expected to adopt automation tools to streamline regulatory processes, which further underscores the importance of a regulatory compliance consultant in addressing these challenges. A transformative case study illustrates AVS Life Sciences' successful enhancement of a biotechnology GMP facility, showcasing their proficiency as a regulatory compliance consultant in quality assurance. This partnership allowed the client to focus on drug development while AVS acted as a regulatory compliance consultant to ensure the facility met stringent compliance standards.
The life sciences oversight and compliance market is anticipated to grow at a rate of 7-9% from 2025 to 2030, reflecting the increasing need for these essential services. As Dr. Sarah Thompson emphasizes, "You can’t afford to take shortcuts when it comes to following regulations." Even minor deviations can result in significant setbacks.
Identify Your Compliance Needs and Challenges
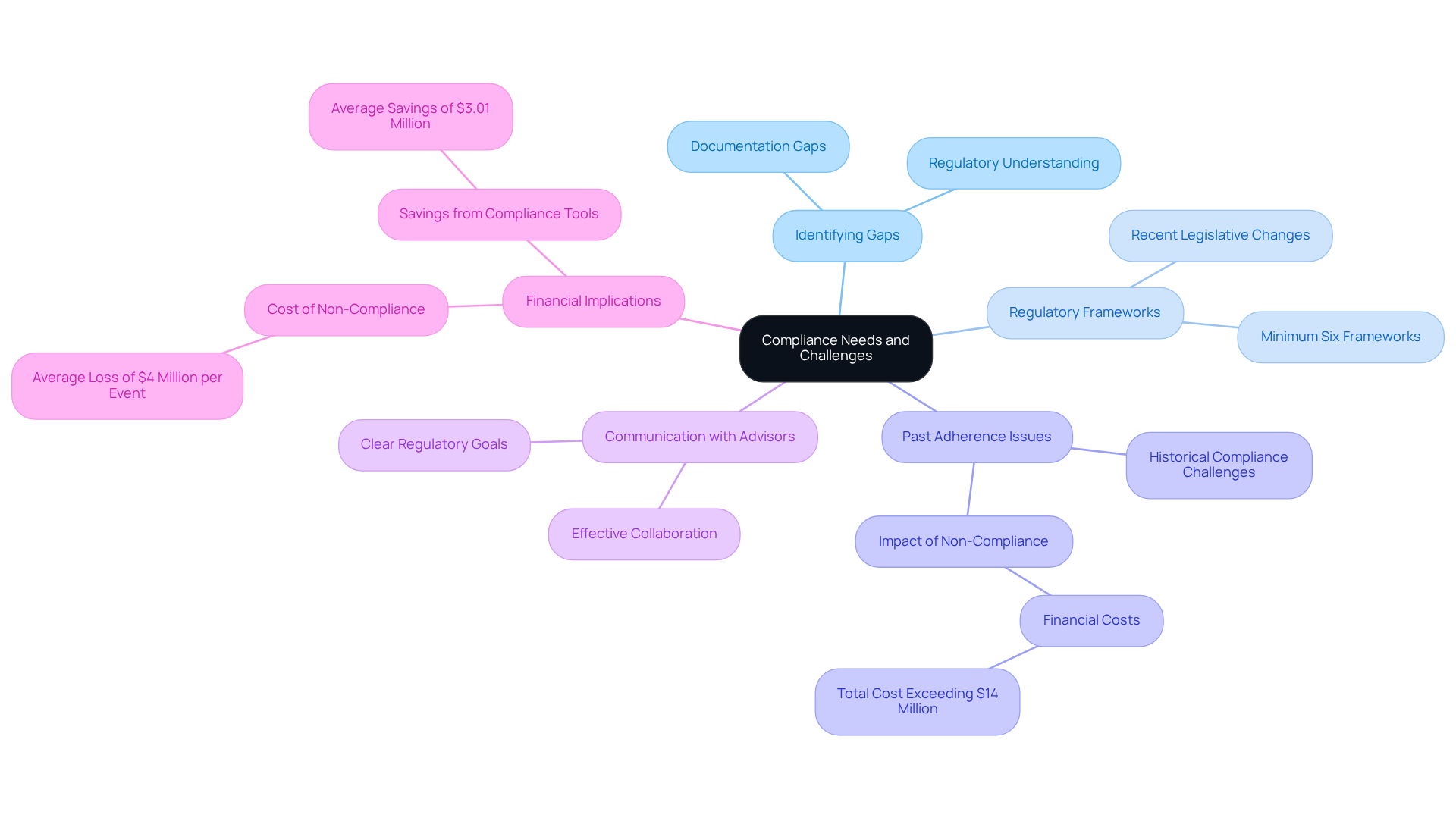
Before hiring a consultant for adherence to regulations, entities must conduct a thorough evaluation of their requirements and obstacles. This essential process involves identifying existing gaps in documentation and understanding the specific regulations applicable to their operations. Recent trends indicate that nearly 70% of service providers must demonstrate adherence to at least six distinct frameworks, underscoring the complexity of the regulatory environment. Companies should assess past adherence issues and consider factors such as the complexity of their products and any recent legislative changes that may impact their regulatory status.
By distinctly outlining their regulatory goals and obstacles, entities can effectively communicate their requirements to prospective advisors, fostering a more productive collaboration. This proactive approach not only aids in recognizing governance challenges but also empowers entities to navigate the more adeptly.
As noted by Sharavanan, "Organizations need to leverage their technology and best practices to enhance efficiency and effectiveness." Furthermore, the financial repercussions of non-compliance are substantial, with estimates suggesting that the total cost of non-compliance can exceed $14 million.
For tailored consulting solutions, organizations can refer to the user manuals provided by AVS Life Sciences, which outline best practices and strategies for effective regulatory management.
Select the Right Regulatory Compliance Consultant
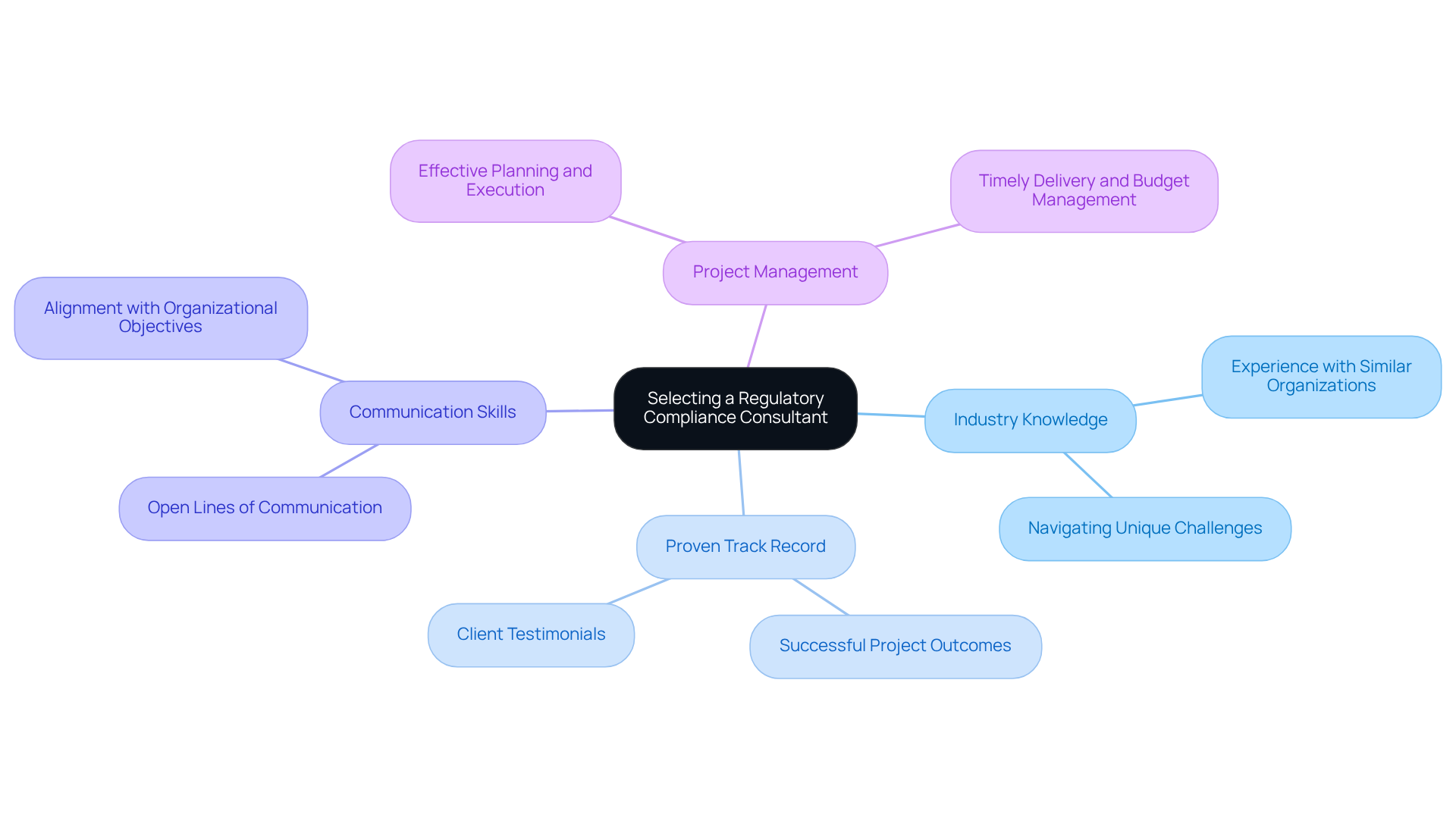
Choosing the appropriate regulatory compliance consultant necessitates a thorough assessment of critical elements. Organizations must prioritize a regulatory compliance consultant who possesses extensive industry knowledge and a proven track record in the specific compliance areas pertinent to their operations. Evaluating their experience with similar organizations and their capability to navigate the unique challenges of the life sciences sector is essential. Notably, over 40% of life science consulting firms identify compliance uncertainty as the most significant barrier to the successful development of combination therapies, emphasizing the importance of a regulatory compliance consultant's specialized expertise.
AVS Life Sciences exemplifies this expertise, having successfully assisted a leading biotechnology company in upgrading their manufacturing facility from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. This project was completed on time and within budget, showcasing AVS's unwavering commitment to quality management and the expertise of a regulatory compliance consultant. The collaboration enabled the client to concentrate on developing medicines for serious diseases, illustrating the transformative impact of effective consulting.
Furthermore, organizations should inquire about the advisor's approach to communication and project management. Effective advisors not only deliver results but also cultivate open lines of communication, ensuring alignment with organizational objectives. Reference checks and case studies, such as AVS's successful GMP facility upgrade, provide valuable insights into the advisor's effectiveness and reliability.
Ultimately, the ideal advisor must not only possess the requisite qualifications but also exhibit a . This alignment significantly enhances the likelihood of achieving adherence and operational excellence in a complex oversight environment. Moreover, AVS Life Sciences' impressive 80% repeat business rate highlights the effectiveness of their advisory services, reinforcing the importance of selecting a professional who is committed to achieving results.
Establish Effective Collaboration with Your Consultant
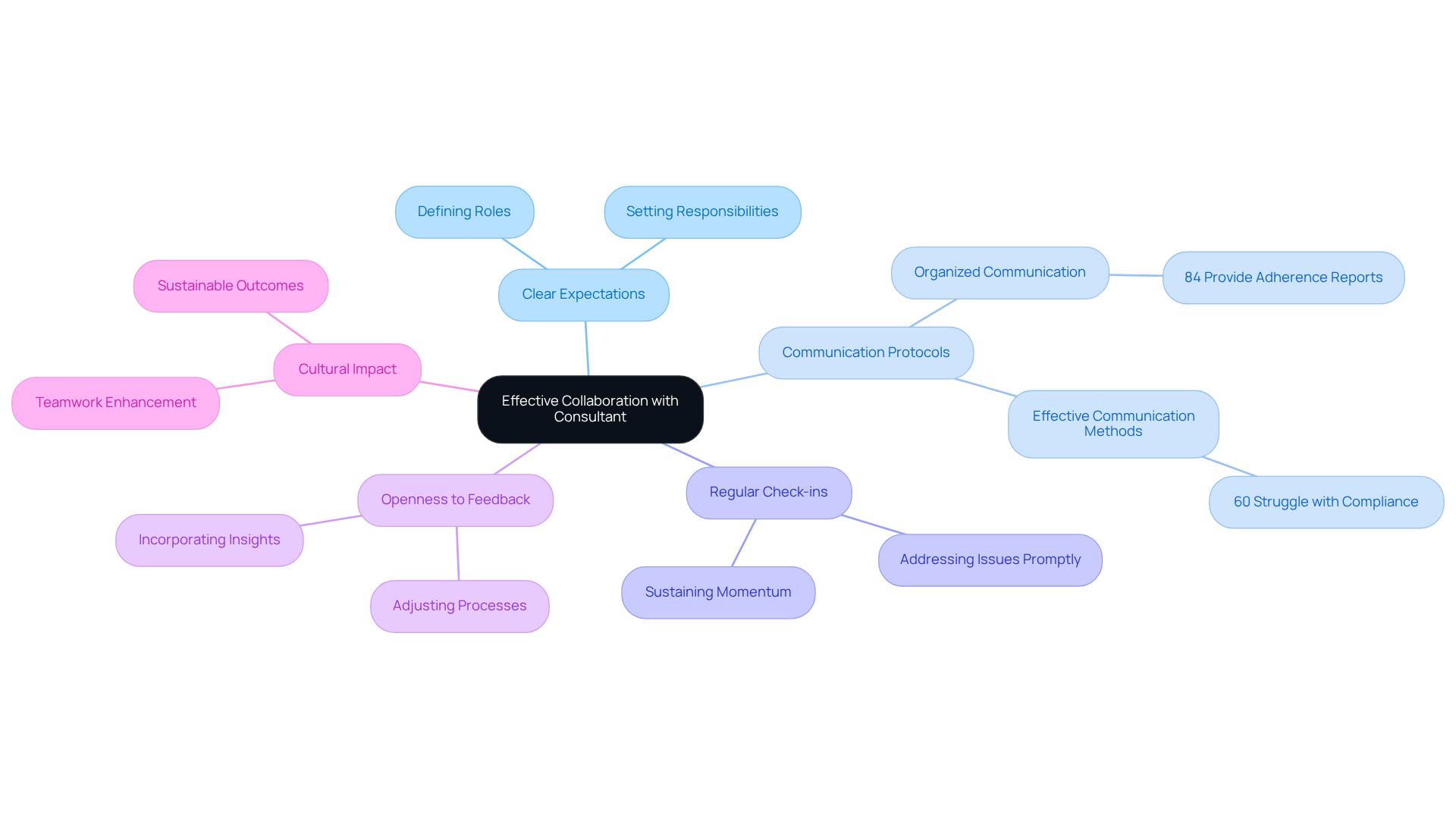
To foster effective collaboration with a regulatory compliance consultant, organizations must establish clear expectations from the outset. This entails defining roles, responsibilities, and communication protocols to ensure alignment on project objectives. Regular check-ins and updates are vital for sustaining momentum and promptly addressing any emerging issues. Organizations should also remain open to feedback and willing to adjust their processes based on the consultant's insights. A culture of openness and teamwork not only enhances the regulatory framework but also yields sustainable outcomes.
Notably, 84% of entities provide reports on their adherence status, underscoring the importance of organized communication in oversight projects. Furthermore, efficient communication methods significantly influence project results, as evidenced by the statistic that 60% of business owners encounter challenges in adhering to rules and regulations.
By prioritizing these practices, entities can leverage the expertise of AVS Life Sciences' advisors, including a regulatory compliance consultant, who specialize in biopharmaceuticals, medical devices, and nutraceuticals, to navigate the complexities of regulatory adherence more effectively. With a pragmatic approach to quality management and , AVS Life Sciences ensures that organizations are well-equipped to meet regulatory standards and achieve sustainable success.
As Paul Koziarz states, 'Without a compliance framework, many organizations wouldn’t have security controls in place, and there would be no consistency of standards among the protocols being used.
Conclusion
Navigating the complexities of regulatory compliance in life sciences is a critical undertaking for organizations aiming to uphold product integrity and patient safety. Selecting the right regulatory compliance consultant can significantly impact an organization’s ability to meet stringent regulations and mitigate the financial risks associated with non-compliance. By understanding the essential steps in choosing a consultant, organizations can ensure they are well-equipped to tackle the evolving regulatory landscape.
The article outlines several key points that are vital in this selection process:
- It emphasizes the importance of understanding specific compliance needs and challenges, which allows organizations to communicate effectively with potential consultants.
- It highlights the necessity of choosing a consultant with relevant industry experience and a proven track record in navigating compliance issues.
- Establishing effective collaboration and communication protocols with the consultant is crucial for fostering a productive partnership that drives sustainable outcomes.
Ultimately, the significance of regulatory compliance consulting in life sciences cannot be overstated. As the industry continues to evolve, organizations must prioritize building robust compliance frameworks and leveraging expert guidance to navigate regulatory challenges. By implementing these best practices, organizations can not only enhance their operational efficiency but also safeguard their reputation and bottom line in a competitive market. Taking proactive steps toward selecting the right consultant is not just a strategic move; it is an essential investment in the future of their business.
Frequently Asked Questions
What is the role of a regulatory compliance consultant in life sciences?
A regulatory compliance consultant in life sciences helps organizations navigate complex regulations, including Good Manufacturing Practices (GMP), ISO standards, and Quality System Regulations (QSR), ensuring compliance and enhancing operational efficiency.
What services does AVS Life Sciences offer in regulatory compliance consulting?
AVS Life Sciences provides comprehensive GXP compliance services, focusing on areas such as GMP audits for APIs, drug products, testing facilities, data integrity, and CAPA processes.
Why is regulatory compliance important in the life sciences industry?
Regulatory compliance is crucial for maintaining product integrity, safeguarding patient safety, and mitigating financial risks associated with non-compliance, which can average over 5 million USD for organizations facing significant issues.
How do regulatory compliance consultants contribute to organizational success?
They assist in establishing robust regulatory frameworks aligned with industry best practices, fostering a culture of quality and accountability within organizations.
What is the projected trend for Quality Management Systems (QMS) in the pharmaceutical industry?
It is projected that over 85% of pharmaceutical companies will implement Quality Management Systems (QMS) by 2025, highlighting the growing importance of regulatory compliance consultants.
What recent trends are affecting regulatory compliance in life sciences?
Recent trends indicate that 78% of organizations are expected to adopt automation tools to streamline regulatory processes, emphasizing the critical role of regulatory compliance consultants in addressing these challenges.
Can you provide an example of a successful regulatory compliance consulting case?
A transformative case study illustrated AVS Life Sciences' successful enhancement of a biotechnology GMP facility, where they ensured compliance standards were met, allowing the client to focus on drug development.
What is the expected growth rate of the life sciences oversight and compliance market?
The life sciences oversight and compliance market is anticipated to grow at a rate of 7-9% from 2025 to 2030, reflecting an increasing need for regulatory compliance services.
What caution does Dr. Sarah Thompson provide regarding regulatory compliance?
Dr. Sarah Thompson emphasizes that shortcuts in following regulations are unacceptable, as even minor deviations can lead to significant setbacks in compliance.
