4 Key Regulatory Consulting Services for Compliance Success

Overview
In the pharmaceutical sector, four key regulatory consulting services are essential for achieving compliance success:
- Expertise in Good Manufacturing Practices (GMP)
- Adherence to ISO standards
- Quality System Regulations (QSR)
- Continuous monitoring of evolving compliance requirements
Engaging specialized consulting services not only enhances organizational compliance capabilities but also mitigates risks and fosters collaboration between compliance teams and regulatory experts. This strategic partnership ultimately leads to improved product quality and strict adherence to regulatory standards, underscoring the necessity of expert guidance in navigating complex compliance landscapes.
Introduction
In the intricate world of pharmaceuticals, regulatory compliance is not merely a necessity; it is a cornerstone of operational integrity and market success. As organizations navigate an ever-evolving landscape of stringent regulations, the role of regulatory consulting services becomes increasingly vital.
These services offer specialized expertise that can transform compliance challenges into strategic advantages. However, how can companies effectively harness these consulting services to not only meet but exceed regulatory expectations?
This article delves into the essential regulatory consulting services that pave the way for compliance success. It reveals best practices and strategies designed to empower organizations to thrive in a complex regulatory environment.
Understand the Role of Regulatory Consulting in Pharmaceutical Compliance
Regulatory consulting services are pivotal in the pharmaceutical sector, providing specialized expertise and guidance to ensure compliance with stringent regulations. AVS Life Sciences stands as your dedicated partner in delivering comprehensive compliance and quality solutions, excelling in the complexities of:
- Good Manufacturing Practices (GMP)
- International Organization for Standardization (ISO) standards
- Quality System Regulations (QSR)
For instance, AVS Life Sciences successfully assisted a leading biotechnology company in elevating their manufacturing facility from a Biosafety Level 1 GMP environment to a Level 2 GMP facility. This transformation not only ensured that all documentation was meticulously organized but also that processes adhered to regulatory standards. Such a proactive strategy mitigates risks and significantly enhances the overall quality of products and services provided by the organization.
Client testimonials consistently highlight the effectiveness of AVS's consultants in managing regulation-driven projects, reinforcing their status as essential partners in achieving and maintaining compliance. Furthermore, the recent acquisition of ValidPath enhances AVS's capabilities in quality engineering and compliance, reflecting the evolving landscape of regulatory consulting services. AVS Life Sciences also extends its expertise across various domains, including medical devices and nutraceuticals, ensuring a holistic approach to compliance consulting.
Implement Best Practices for Engaging Regulatory Consultants
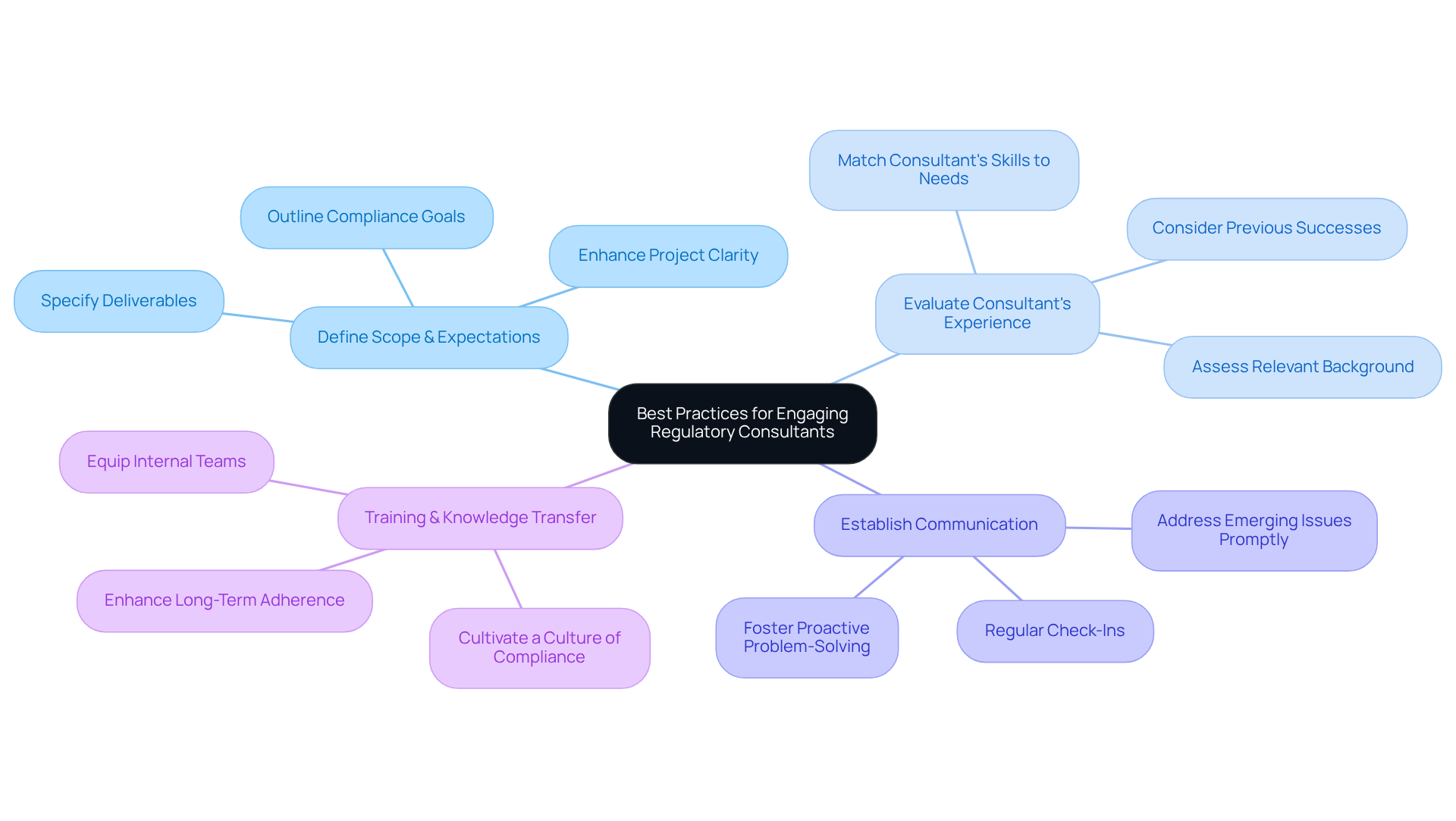
To fully harness the advantages of engaging regulatory consulting services, entities must adhere to several best practices. First and foremost, it is essential to clearly define the scope of work and expectations prior to initiating the partnership. This involves outlining specific compliance goals and deliverables, which significantly enhances project clarity and focus. Statistics reveal that a well-defined scope of work is critical in consulting partnerships, as it establishes the foundation for successful collaboration.
Next, entities should rigorously evaluate the consultant's experience and knowledge in regulatory consulting services related to relevant fields. For instance, a consultant with a robust background in FDA submissions is likely to be more effective for a company aiming to launch a new drug. This focused selection procedure ensures that the consultant's capabilities align with the entity's particular requirements for regulatory consulting services.
Establishing open lines of communication is vital for facilitating collaboration and ensuring alignment throughout the project. Regular check-ins and updates can promptly address any emerging issues, fostering a proactive approach to problem-solving.
Lastly, entities should consider the consultant's approach to training and knowledge transfer when utilizing regulatory consulting services. Equipping internal teams with regulatory knowledge not only enhances immediate adherence efforts but also contributes to long-term success. By investing in the growth of internal capabilities, entities can cultivate a lasting culture of adherence that endures beyond the consulting engagement.
To discover more about how AVS Life Sciences can assist you in achieving your regulatory objectives, contact us today for customized consulting solutions. It is crucial to recognize that failing to clearly outline expectations can lead to miscommunication and project delays, underscoring the importance of a well-defined scope of work.
Adapt Regulatory Strategies to Evolving Compliance Requirements
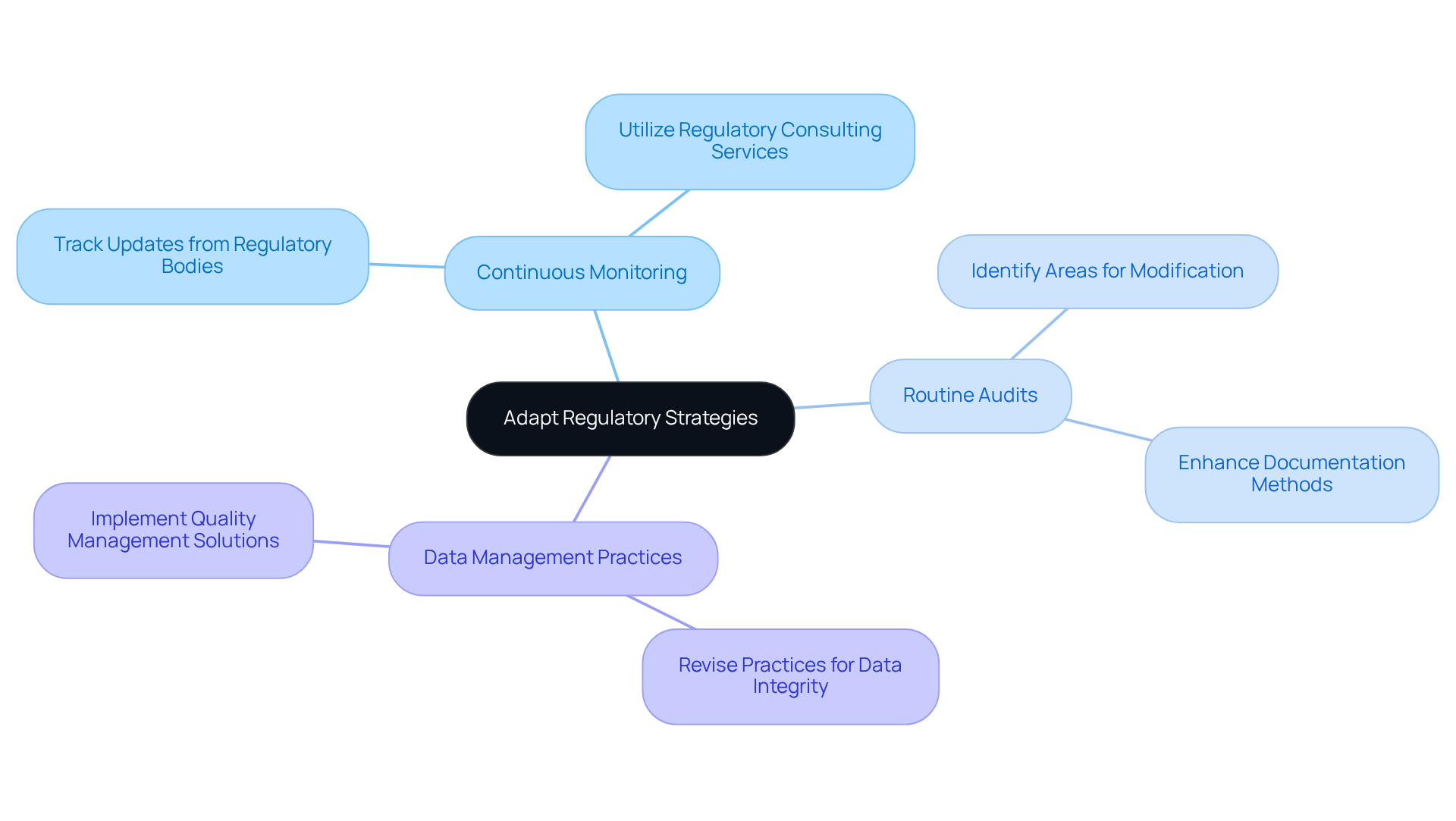
In the rapidly evolving landscape of pharmaceutical regulations, organizations face significant compliance challenges that demand agility in their regulatory consulting services. Staying informed about compliance changes, including GXP and FDA regulations, is crucial for regulatory consulting services to understand their implications on existing processes.
To address this, companies should implement a continuous monitoring system to track updates from regulatory bodies, with the assistance of regulatory consulting services for agencies like the FDA and EMA. Additionally, performing routine audits can help pinpoint areas needing modifications, particularly in documentation methods and data integrity oversight, which may require regulatory consulting services.
For example, should new guidelines regarding data integrity be introduced, a company must revise its data management practices accordingly. With the U.S. pharmaceutical market projected to exceed $1 trillion by 2030, the necessity of adapting regulatory consulting services becomes increasingly clear.
By fostering a culture of flexibility and leveraging comprehensive quality management solutions from AVS Life Sciences, organizations can ensure compliance while enhancing operational efficiency, thereby positioning themselves for success in a complex oversight environment.
Foster Collaboration Between Compliance Teams and Regulatory Experts
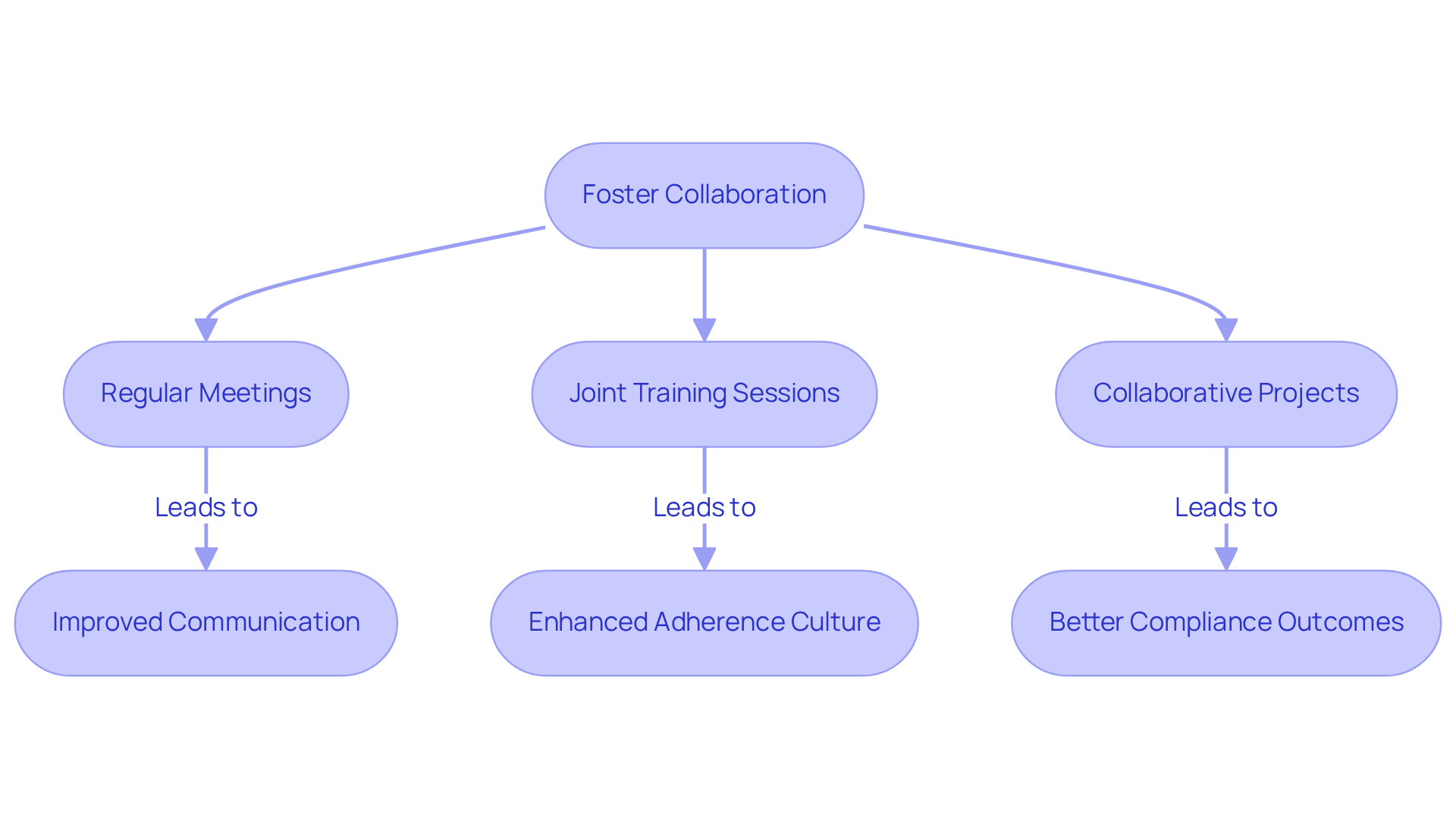
Cooperation between adherence teams and oversight specialists is essential for achieving success in meeting compliance requirements through regulatory consulting services. Promoting cross-functional collaboration allows professionals to work closely with advisory consultants, facilitating the exchange of insights and best practices. This collaboration can be strengthened through:
- Regular meetings
- Joint training sessions
- Collaborative projects
For instance, a governance group might partner with a legal advisor to create a comprehensive training initiative that aligns legal standards with internal protocols. Such initiatives not only foster a cooperative atmosphere but also enhance the adherence culture within organizations, improve communication, and yield more favorable oversight outcomes.
Data indicates that organizations employing cross-functional collaboration in their regulatory consulting services realize significantly higher success rates, underscoring the importance of this approach within the pharmaceutical sector. A recent case study exemplifies this: AVS Life Sciences collaborated with a leading biotechnology company to upgrade their GMP facility. This partnership encountered challenges, including discrepancies in test results due to improperly installed barcode scanner cameras. By addressing these issues and capturing valuable lessons learned, AVS Life Sciences enabled the client to improve their quality control processes, ensuring compliance with regulatory standards.
Conclusion
Navigating the complexities of pharmaceutical compliance is essential for organizations aiming to thrive in a highly regulated environment. Engaging regulatory consulting services not only provides specialized expertise but also fosters a proactive approach to meeting compliance requirements. By leveraging the insights and capabilities of firms like AVS Life Sciences, companies can ensure they remain compliant while enhancing the quality and efficiency of their operations.
The article highlights several key aspects of successful regulatory consulting:
- Defining clear project scopes is paramount.
- Selecting consultants with relevant experience is crucial.
- Fostering open communication is essential.
- Adapting regulatory strategies to evolving compliance requirements is important.
- Promoting collaboration between compliance teams and regulatory experts is vital for achieving sustained success.
These practices empower organizations to navigate the regulatory landscape effectively, ensuring they are well-prepared for any challenges that may arise.
Ultimately, the significance of regulatory consulting in the pharmaceutical industry cannot be overstated. As compliance requirements continue to evolve, embracing best practices and fostering collaboration will not only enhance adherence but also cultivate a culture of continuous improvement. Organizations are encouraged to prioritize these strategies and consider how they can integrate regulatory consulting services into their operations to achieve lasting compliance success.
Frequently Asked Questions
What is the role of regulatory consulting in the pharmaceutical sector?
Regulatory consulting provides specialized expertise and guidance to ensure compliance with stringent regulations in the pharmaceutical sector.
What specific areas do regulatory consulting services cover?
Regulatory consulting services cover areas such as Good Manufacturing Practices (GMP), International Organization for Standardization (ISO) standards, and Quality System Regulations (QSR).
Can you provide an example of regulatory consulting in action?
AVS Life Sciences assisted a leading biotechnology company in upgrading their manufacturing facility from a Biosafety Level 1 GMP environment to a Level 2 GMP facility, ensuring documentation was organized and processes adhered to regulatory standards.
How do regulatory consulting services benefit organizations?
These services mitigate risks and significantly enhance the overall quality of products and services provided by organizations.
What do client testimonials say about AVS Life Sciences?
Client testimonials highlight the effectiveness of AVS's consultants in managing regulation-driven projects, reinforcing their status as essential partners in achieving and maintaining compliance.
How has AVS Life Sciences expanded its capabilities?
The recent acquisition of ValidPath has enhanced AVS's capabilities in quality engineering and compliance.
In which additional domains does AVS Life Sciences provide expertise?
AVS Life Sciences extends its expertise across various domains, including medical devices and nutraceuticals, ensuring a holistic approach to compliance consulting.
