4 Key Practices in Regulatory Consulting for Compliance Officers

Overview
The article delineates four pivotal practices in regulatory consulting for compliance officers:
- Understanding regulatory frameworks
- Developing tailored compliance strategies
- Maintaining comprehensive documentation
- Implementing continuous training programs
These practices are not merely procedural; they are essential for ensuring adherence to industry standards and regulations. By embracing these strategies, organizations can effectively identify gaps in compliance, foster accountability among their teams, and enhance the knowledge and skills of their regulatory personnel in a rapidly evolving landscape. This proactive approach not only mitigates risks but also positions organizations to thrive amidst regulatory challenges.
Introduction
Navigating the intricate world of regulatory compliance presents a formidable challenge for compliance officers, particularly as industries evolve and standards shift. Mastering key practices in regulatory consulting empowers these professionals to safeguard their organizations against potential pitfalls while enhancing operational integrity. What essential strategies can transform compliance efforts from a reactive obligation into a proactive advantage? This article explores four pivotal practices that not only streamline adherence but also cultivate a culture of continuous improvement in the face of ever-changing regulations.
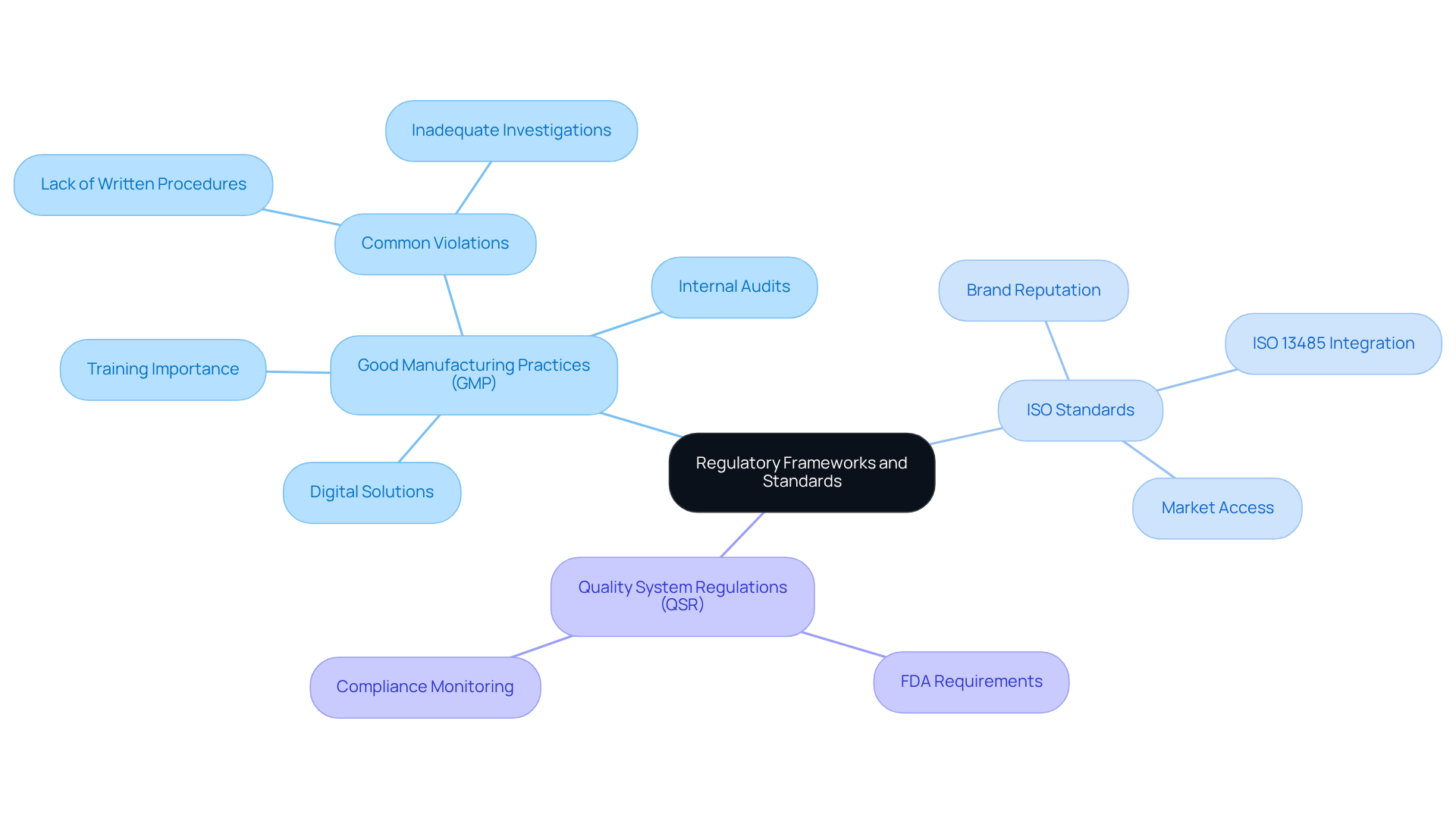
Understand Regulatory Frameworks and Standards
Compliance officers must possess a thorough understanding of the frameworks that oversee their industry, including:
Each of these frameworks specifies particular criteria that organizations must fulfill, making it crucial for officers to identify possible gaps in adherence. The recent integration of , effective February 2026, underscores the necessity for companies to to .
Staying informed about the latest changes in regulations is essential, as it can prevent costly penalties and enhance operational efficiency. Notably, FDA inspections for medical devices rose by 144% in 2023 compared to the prior year, highlighting the increasing scrutiny on adherence. Routine skill-building sessions and workshops are vital for keeping oversight teams updated on these developments. Practical instances demonstrate that significantly reduces the risk of violations; organizations that prioritize ongoing education are better equipped to navigate complex oversight environments.
Moreover, the cannot be overstated. These standards not only facilitate market access but also safeguard brand reputation by ensuring product quality and safety. As the pharmaceutical industry evolves, for the adoption of these standards and the promotion of a culture of compliance to maintain operational integrity and achieve long-term success.
Develop Tailored Compliance Strategies
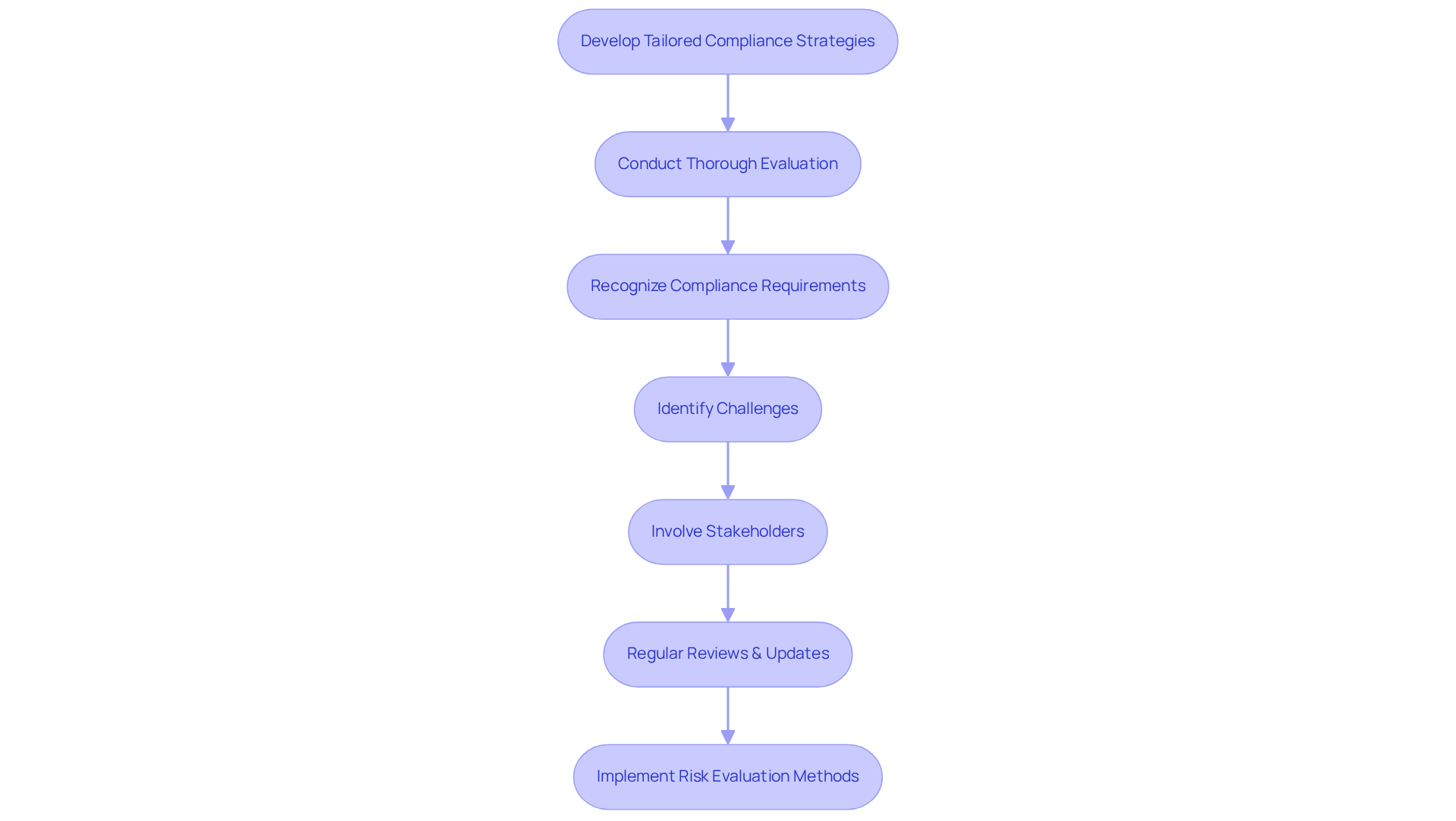
To develop effective adherence strategies, officers must conduct a thorough evaluation of their organization's distinct requirements and risks. This process begins with recognizing relevant to operations, such as stringent for pharmaceutical companies or . A significant instance is of a biotechnology GMP facility from Biosafety Level 1 to Level 2, underscoring the critical in the industry.
During this upgrade, challenges such as the misalignment of barcode scanner cameras were identified, which resulted in unreliable test results. Involving stakeholders from various departments is essential, as it provides valuable perspectives on potential in the development of robust strategies. Furthermore, regular reviews and updates of these strategies are crucial to ensure they remain relevant and effective in the context of and navigating the ever-evolving regulatory landscape.
As regulatory professionals face increasing pressures, including the necessity for real-time monitoring and adapting to new regulations, a is essential for success in 2025 and beyond. For instance, biotechnology companies can implement risk evaluation methods like scenario analysis and stress testing to identify weaknesses in their regulatory frameworks. Additionally, adhering to guidelines for can further enhance regulatory efforts.
By avoiding common pitfalls, such as neglecting to regulatory shifts, organizations can significantly improve their adherence to standards and achieve better outcomes.
Maintain Comprehensive Documentation and Audit Trails
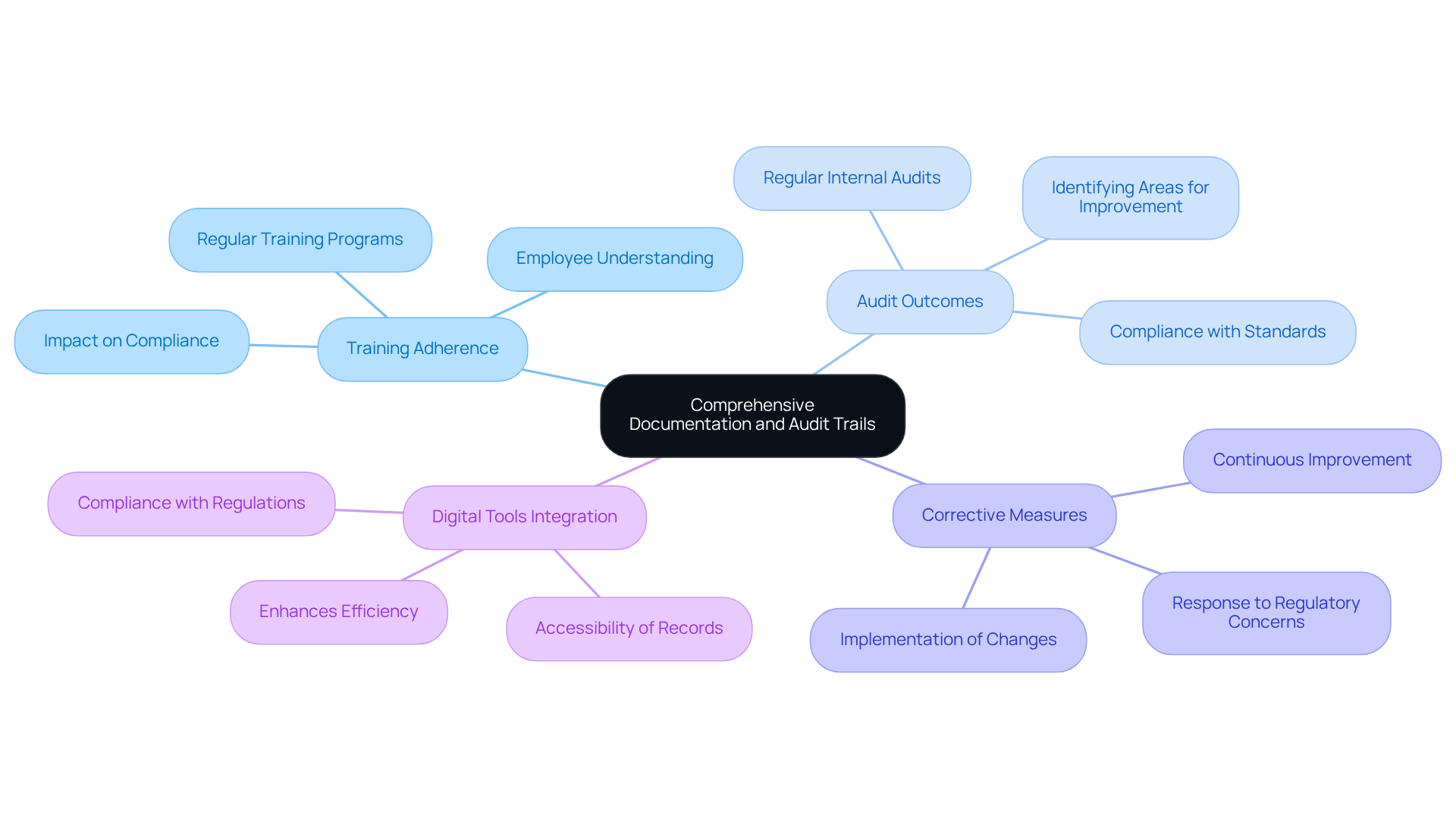
Compliance officers must prioritize that meticulously capture all . This includes maintaining thorough documentation of:
- Audit outcomes
- in response to concerns
Creating comprehensive is crucial; these trails must record every action performed within the , ensuring transparency and accountability. In 2025, the integration of digital tools and software will be increasingly vital, enhancing the efficiency of documentation processes and ensuring that records are easily accessible and up-to-date.
Regular are essential for identifying areas for improvement and ensuring adherence to the standards set by regulatory consulting. As noted by industry experts, effective audit trails not only support regulations but also foster a culture of accountability within organizations, ultimately safeguarding product quality and patient safety.
Implement Continuous Training and Education Programs
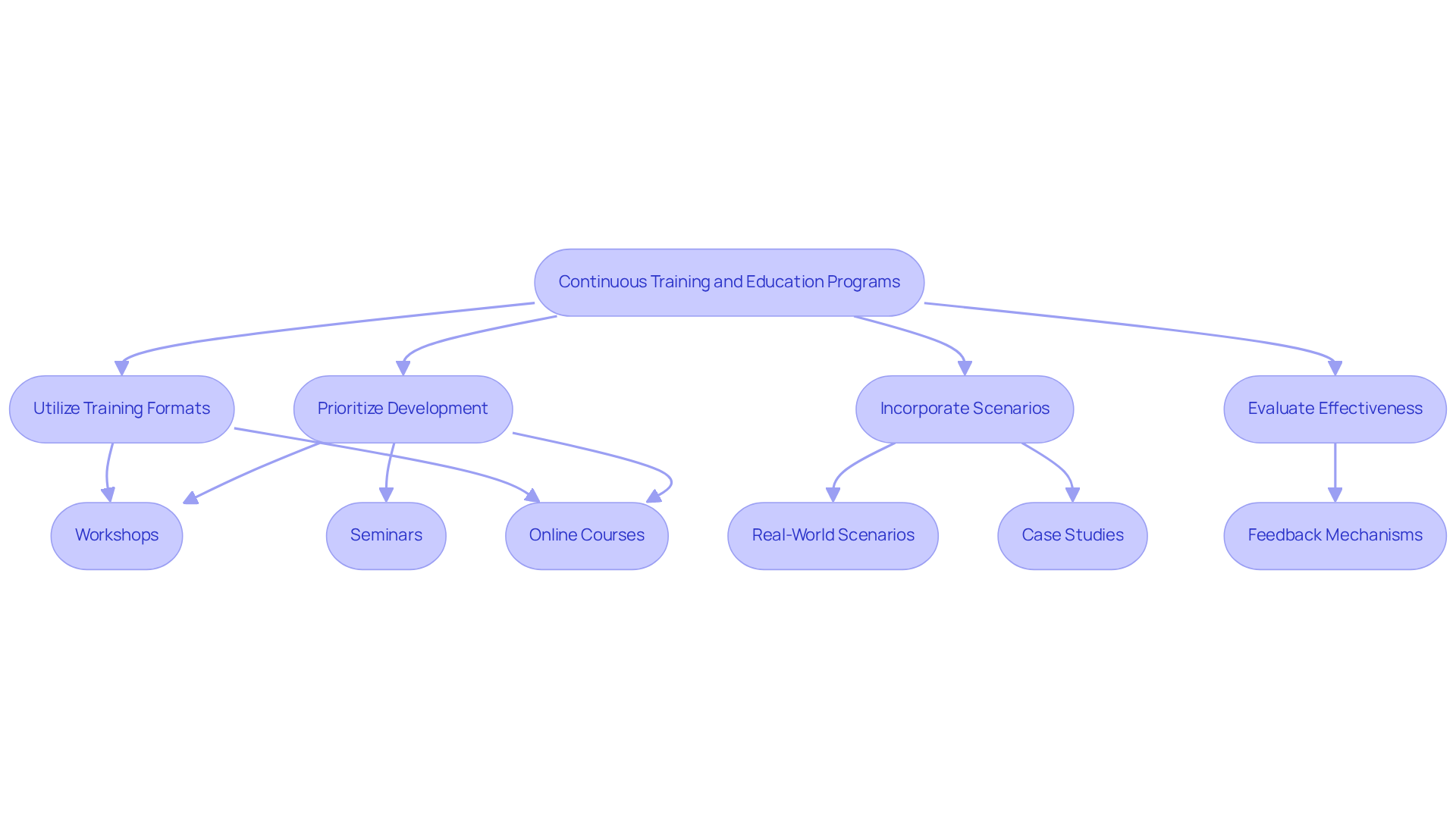
Organizations must prioritize ongoing development and education initiatives for their regulatory teams in the area of , particularly in the rapidly evolving pharmaceutical industry. Regular workshops, online courses, and seminars focusing on the latest regulatory developments and , such as the FDA's Guidance, are essential for . Grasping this advice is crucial, as numerous organizations continue to encounter citations for data management problems, highlighting the necessity for thorough education on the significance of data integrity in business decision-making and data documentation.
Incorporating real-world scenarios and case studies into learning sessions has proven effective in enhancing engagement and knowledge retention. A significant instance is the EDGE development initiative executed by Allied Universal, which has effectively provided security experts with crucial information. This initiative has led to a decrease in safety occurrences and , thus boosting adherence metrics.
Promoting a through open communication and feedback is essential, as it encourages employees to take ownership of their responsibilities. Studies suggest that 76% of employees are more inclined to remain at companies that provide , highlighting the importance of investing in [regulatory consulting](https://trainingindustry.com/articles/performance-management/top-notch-10-statistics-that-highlight-the-significance-of-continuous-training) specifically. Furthermore, evaluating the effectiveness of training programs through assessments and feedback mechanisms can help refine these initiatives, ensuring they meet the evolving needs of the organization. As industry leaders emphasize, continuous learning not only mitigates risks but also enhances overall organizational performance, making it an essential component of a robust regulatory consulting strategy.
Conclusion
Compliance officers occupy a crucial position in guiding organizations through the intricate landscape of regulatory requirements. By mastering key practices such as:
- Understanding regulatory frameworks
- Developing tailored compliance strategies
- Maintaining thorough documentation
- Prioritizing continuous training
compliance professionals can significantly bolster their organizations' adherence to regulations.
The article underscores the necessity of remaining informed about evolving regulatory standards and adapting compliance strategies to meet distinct organizational needs. Regular training and robust documentation practices not only cultivate a culture of accountability but also equip organizations for the heightened scrutiny from regulatory bodies. As emphasized, the integration of digital tools and ongoing education initiatives are vital for fostering a proactive compliance environment.
Investing in these best practices transcends mere regulatory obligation; it embodies a strategic approach that safeguards product quality, enhances operational efficiency, and protects brand reputation. As the regulatory landscape continues to evolve, compliance officers must wholeheartedly adopt these practices to ensure long-term success and resilience within their organizations.
Frequently Asked Questions
What are the key regulatory frameworks that compliance officers need to understand?
Compliance officers must understand Good Manufacturing Practices (GMP), International Organization for Standardization (ISO) standards, and Quality System Regulations (QSR).
Why is it important for compliance officers to identify gaps in adherence to these frameworks?
Identifying gaps in adherence is crucial because it helps organizations fulfill specific criteria required by these frameworks, preventing costly penalties and enhancing operational efficiency.
What significant change regarding ISO standards is set to take place in February 2026?
The integration of ISO 13485 into FDA regulations will take effect in February 2026, necessitating companies to align with global standards for compliance.
How has the frequency of FDA inspections for medical devices changed recently?
FDA inspections for medical devices increased by 144% in 2023 compared to the previous year, indicating heightened scrutiny on adherence to regulations.
What role do skill-building sessions and workshops play for compliance officers?
Routine skill-building sessions and workshops are vital for keeping oversight teams updated on the latest regulatory developments and ensuring they are equipped to navigate complex oversight environments.
How does effective GMP training impact organizations?
Effective GMP training significantly reduces the risk of violations, helping organizations that prioritize ongoing education to better navigate regulatory challenges.
What is the significance of ISO standards in regulatory compliance?
ISO standards facilitate market access and safeguard brand reputation by ensuring product quality and safety, making them essential for compliance in the pharmaceutical industry.
Why is regulatory consulting important in the pharmaceutical industry?
Regulatory consulting is critical for the adoption of ISO standards and the promotion of a culture of compliance, which helps maintain operational integrity and achieve long-term success.
