4 Key Practices for Safe Parenteral Injection Compliance

Overview
Key practices for safe parenteral injection compliance encompass a deep understanding of regulatory standards, the implementation of best safety practices, meticulous documentation, and robust staff training.
Adherence to these practices, bolstered by regulatory frameworks such as Good Manufacturing Practices (GMP) and ongoing education, significantly mitigates the risks associated with parenteral injections while enhancing patient safety outcomes.
By prioritizing these elements, organizations not only comply with industry standards but also foster a culture of safety and excellence.
Introduction
In the intricate landscape of healthcare, the safety and compliance of parenteral injections stand as a critical priority. As biologics gain prominence and regulatory standards evolve, organizations are tasked with the essential duty of adhering to rigorous guidelines while ensuring patient safety. This article explores four fundamental practices that not only bolster compliance but also markedly enhance patient outcomes.
How can healthcare providers adeptly maneuver through the complexities of parenteral injection safety in an era of heightened demands for quality and accountability?
Understand Regulatory Standards for Parenteral Injections
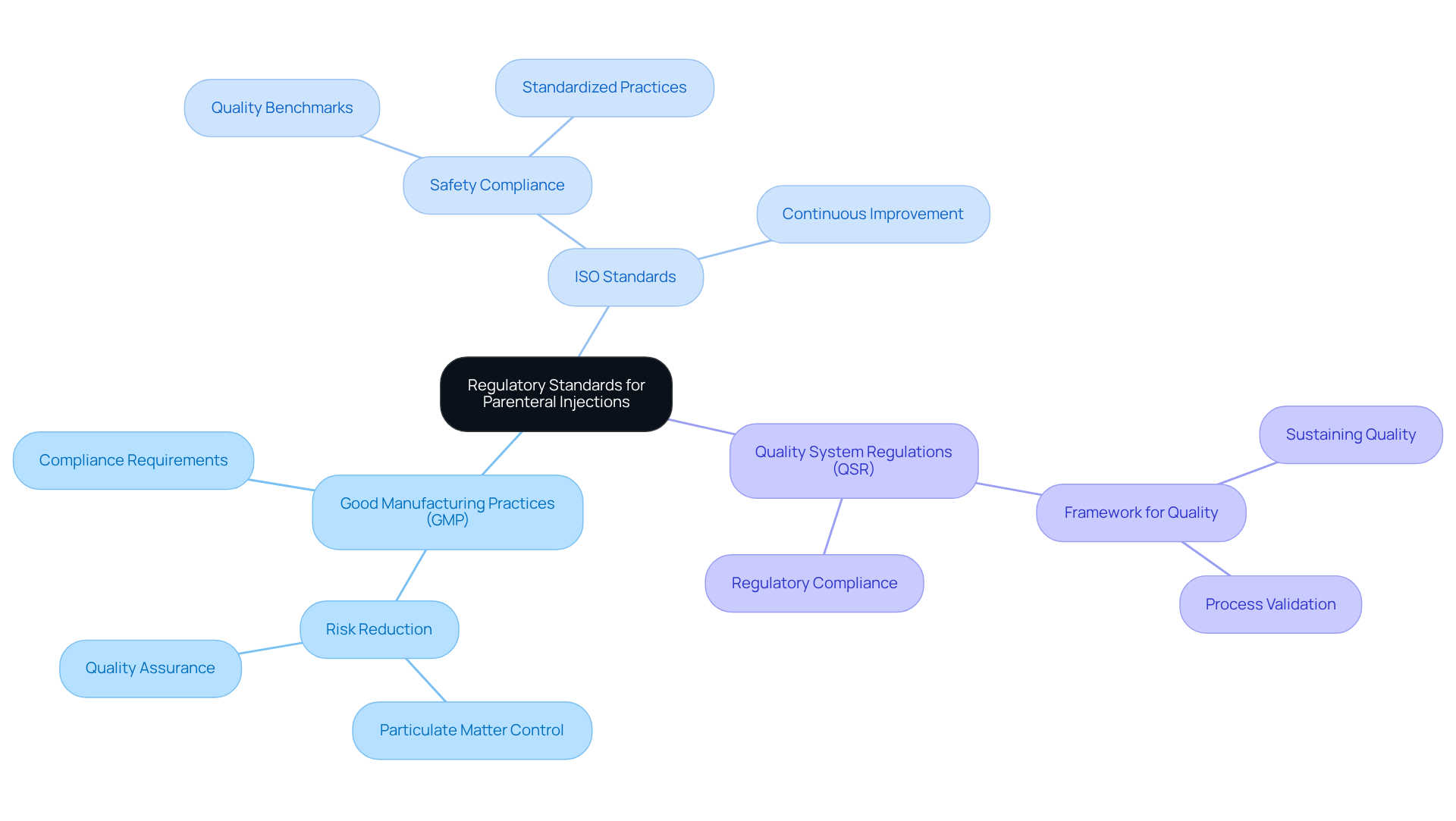
Organizations must have a comprehensive understanding of key regulatory frameworks, including Good Manufacturing Practices (GMP), ISO standards, and Quality System Regulations (QSR), to ensure compliance with parenteral injection standards. These regulations set forth the critical for the manufacturing and administration of injectable medications. It is essential to consistently review updates from oversight organizations such as the FDA and EMA to stay informed about evolving guidelines.
Key Regulatory Frameworks:
- Good Manufacturing Practices (GMP): These are vital for reducing risks associated with particulate matter in injectable formulations, which can originate from excipients, active pharmaceutical ingredients (APIs), or the manufacturing process.
- ISO Standards: These enhance safety compliance by ensuring that practices related to parenteral injection meet stringent quality benchmarks.
- Quality System Regulations (QSR): These provide a framework for sustaining quality throughout the manufacturing process.
For example, a pharmaceutical company that implemented a compliance checklist experienced a 30% reduction in audit findings, underscoring the effectiveness of this proactive approach. As the biologics sector continues to expand, with a projected compound annual growth rate of 15% through 2027, the importance of these regulatory frameworks in safeguarding the integrity and safety of injectable medications cannot be overstated.
AVS Life Sciences offers comprehensive GXP compliance services, which include audits of API and Drug Product CMOs, Contract Test Labs, and other manufacturing and testing facilities. Our expertise in enhancing GMP facilities prioritizes quality assurance and regulatory compliance, enabling our clients to focus on developing safe and effective medications for parenteral injection.
Implement Best Practices for Patient Safety in Parenteral Administration
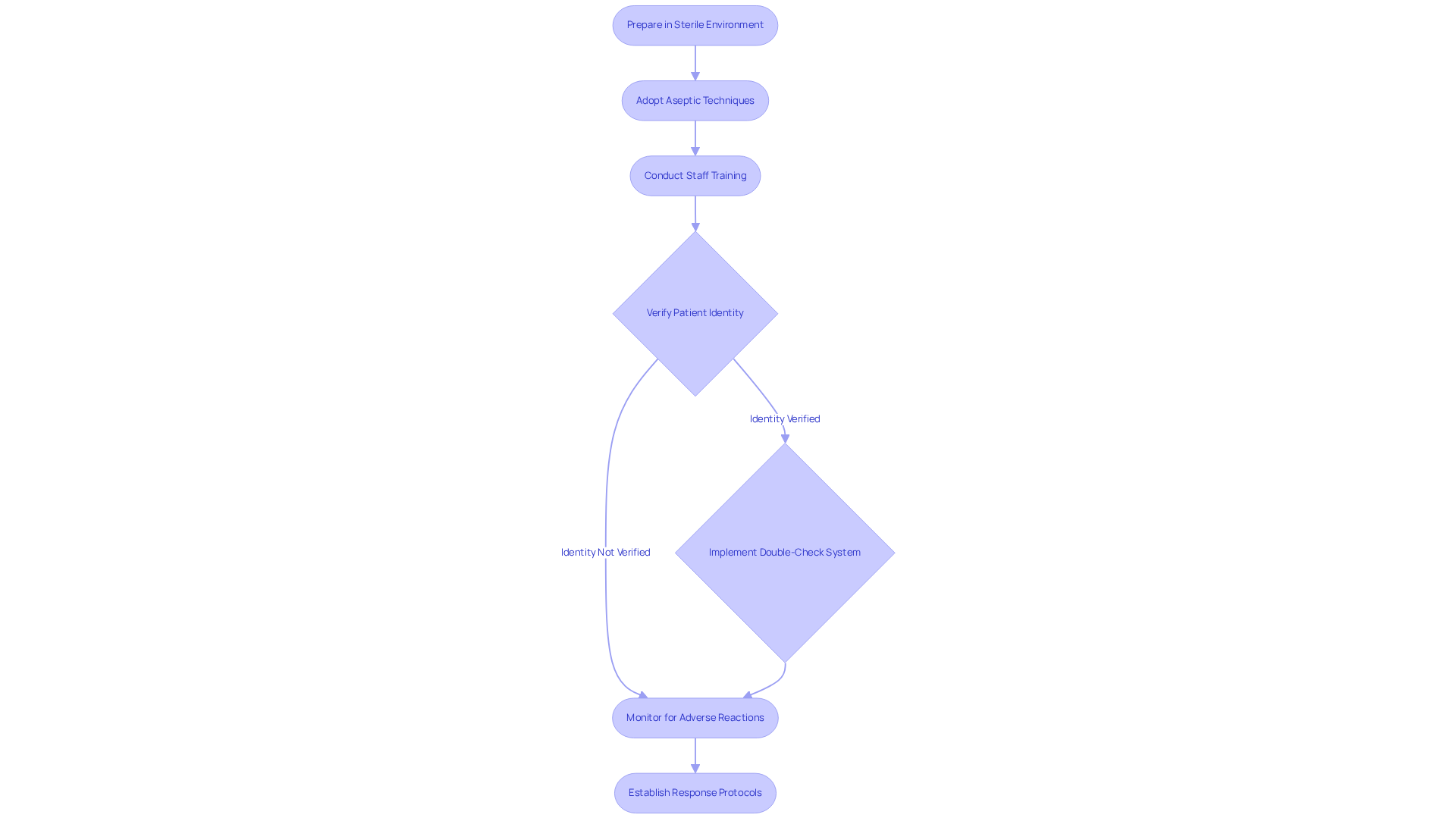
Applying optimal methods for patient safety in parenteral injection necessitates a multifaceted approach. First and foremost, all injections must be prepared in a sterile environment to mitigate contamination risks. Adopting strict aseptic techniques during both preparation and administration is essential; this includes using new sterile syringes and needles for each patient and ensuring that medication preparation areas are free from potential contaminants. Regular training sessions for staff on these protocols are essential to uphold high standards of conduct, in accordance with the CDC's Standard Precautions guidelines designed to prevent healthcare-associated infections.
Additionally, verifying patient identity and medication before administration is vital to prevent errors. Employing a double-check system, where two qualified personnel confirm the medication and dosage, significantly enhances safety. This approach aligns with the CDC's guidelines on preventing healthcare-associated infections, which emphasize the importance of Standard Precautions.
Monitoring patients for adverse reactions post-injection is equally important. Establishing protocols for immediate response to any complications can save lives. A recent case study on 'Preventing Unsafe Injection Methods' demonstrated that by implementing these best approaches, they achieved a remarkable 40% reduction in medication errors, thereby significantly enhancing patient safety. Furthermore, utilizing PCA pumps with integrated capnography can prevent over 60% of adverse events related to PCA pumps, highlighting the importance of vigilant monitoring in the administration of parenteral injection therapies. This emphasizes the essential requirement for and awareness of possible pitfalls in injection methods.
Maintain Comprehensive Documentation and Audit Trails
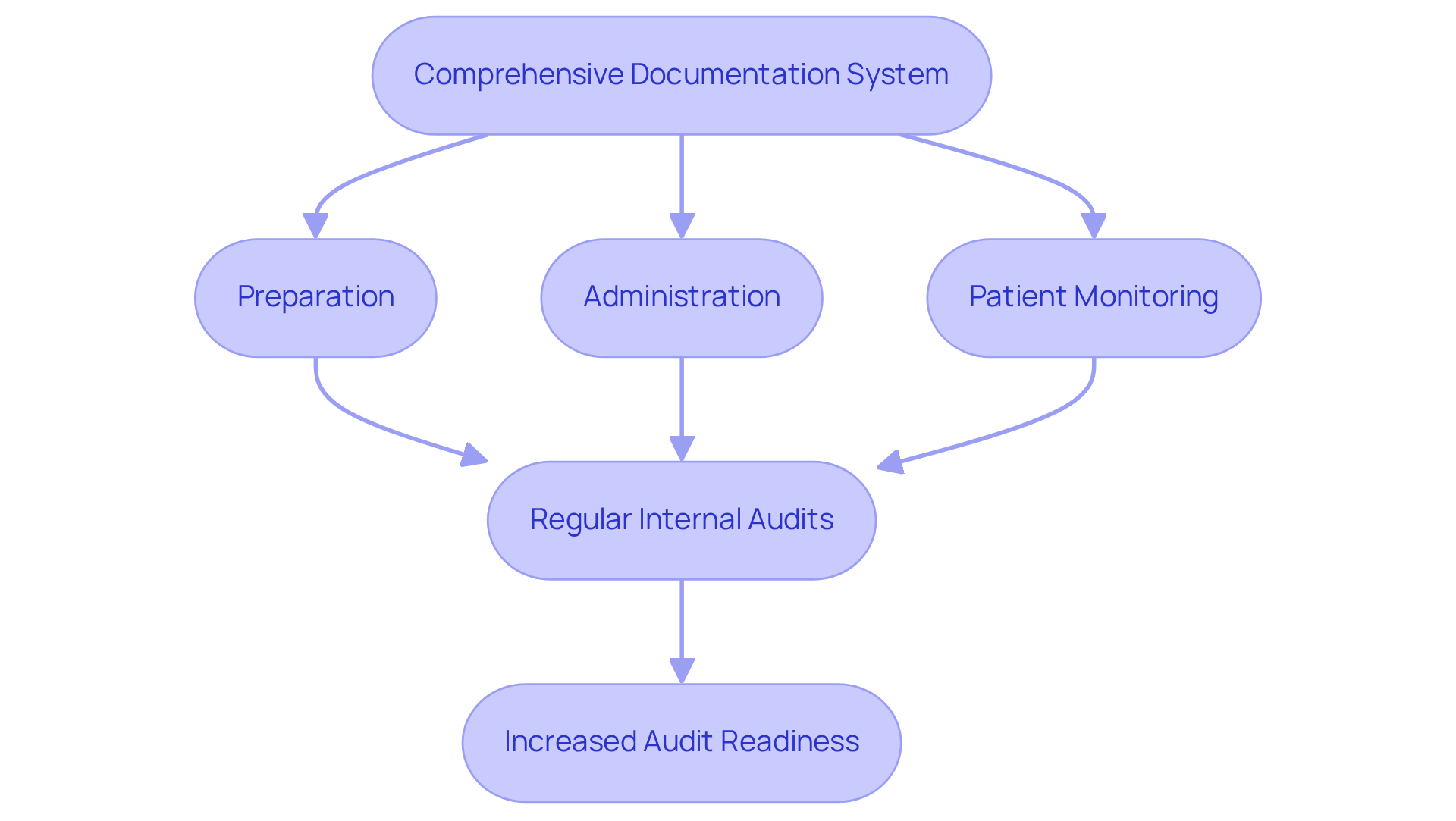
Maintaining comprehensive records and audit trails is vital for compliance in procedures involving parenteral injection. Organizations must implement a robust documentation system that meticulously records each step of the parenteral injection process, encompassing preparation, administration, and patient monitoring. This documentation should be readily and inspections.
Furthermore, it is essential for organizations to conduct regular internal audits to verify adherence to documentation protocols and identify opportunities for improvement. For instance, a pharmaceutical company that established a digital documentation system experienced a remarkable 50% increase in audit readiness, underscoring the effectiveness of diligent record-keeping.
Enhance Staff Training and Continuous Education on Compliance
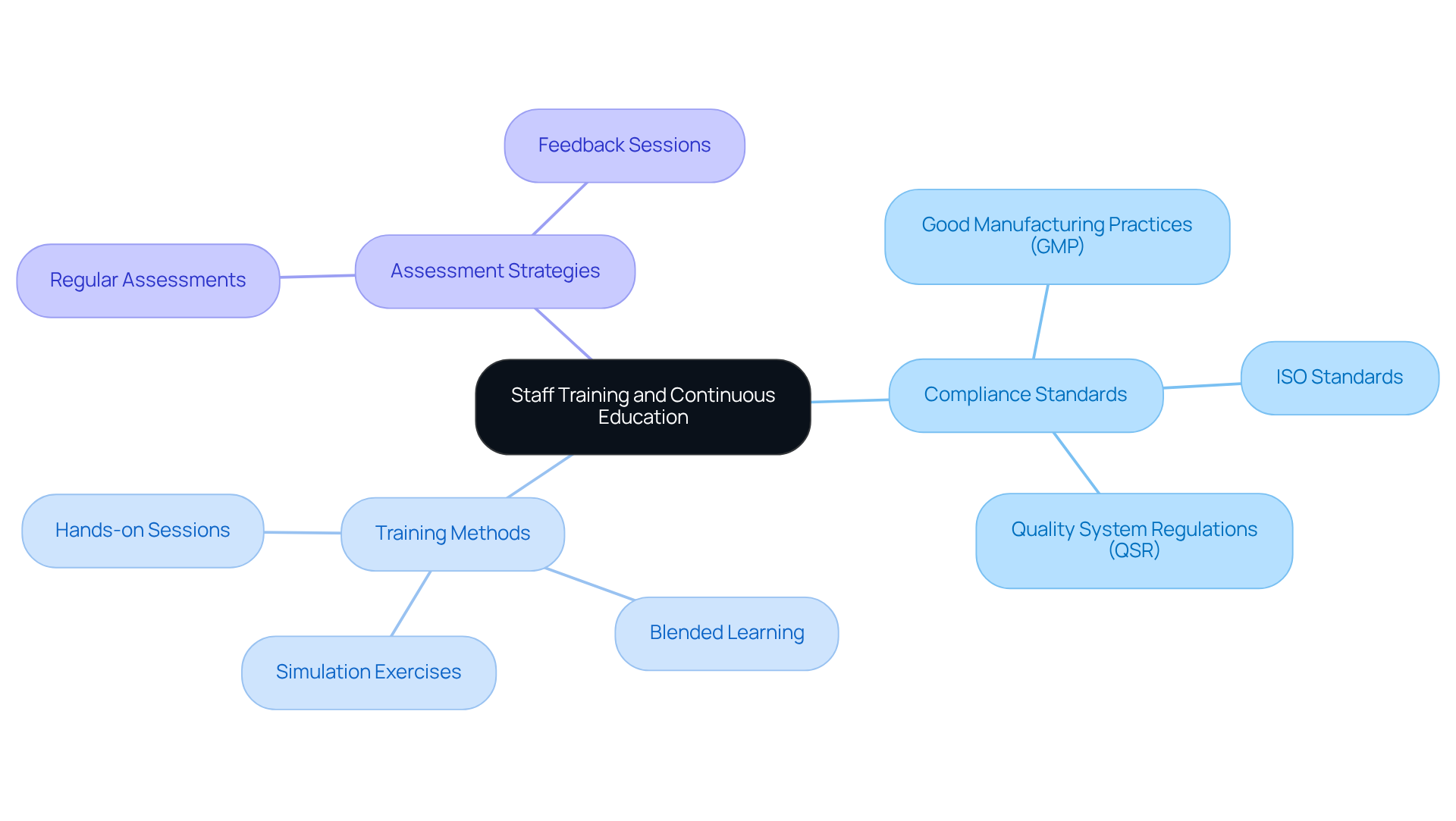
Improving employee development and ongoing education on compliance is essential for upholding high standards in procedures involving parenteral injection. Organizations must establish a comprehensive development program that encompasses initial instruction for new hires and continuous education for current staff. This program should thoroughly address compliance standards, including:
alongside best practices for patient safety and documentation requirements. AVS Life Sciences, a leading provider of quality management and regulatory compliance solutions, underscores the importance of such education in ensuring adherence across APIs, drug products, and testing facilities.
Incorporating simulation exercises allows staff to refine their skills in a controlled environment, significantly enhancing their confidence and competence. A blended learning strategy that integrates various teaching methods, such as hands-on sessions and online modules, can further bolster the effectiveness of the education. Regular assessments and feedback sessions are crucial for reinforcing learning and identifying knowledge gaps. For instance, a healthcare institution that implemented a continuous education program experienced an impressive 25% improvement in adherence scores during evaluations, underscoring the value of investing in staff development.
By prioritizing continuous education, healthcare organizations can enhance adherence and ultimately improve patient outcomes. This is exemplified by AVS Life Sciences' successful upgrade of a biotechnology GMP facility, where a and regulatory compliance resulted in significant advancements in operational standards.
Conclusion
Understanding and adhering to key practices for safe parenteral injection compliance is crucial for ensuring patient safety and maintaining regulatory standards. By focusing on regulatory frameworks, best practices in patient safety, comprehensive documentation, and continuous staff training, organizations can significantly enhance their compliance efforts and safeguard the integrity of injectable medications.
The importance of staying informed about regulatory standards such as GMP, ISO, and QSR cannot be overstated; these are essential for quality assurance in parenteral injections. Implementing best practices—including strict aseptic techniques and patient monitoring protocols—further reduces the risk of errors and adverse events. Additionally, maintaining thorough documentation and conducting regular audits are vital for ensuring accountability and compliance. Ongoing staff education ensures that healthcare professionals are well-equipped to uphold these standards.
Ultimately, the commitment to safe parenteral injection practices not only protects patients but also reinforces the integrity of healthcare systems. Organizations are encouraged to prioritize these practices and invest in continuous education and training for their staff. By doing so, they can foster a culture of safety and compliance that ultimately leads to better patient outcomes and a more effective healthcare environment.
Frequently Asked Questions
What are the key regulatory frameworks for parenteral injections?
The key regulatory frameworks for parenteral injections include Good Manufacturing Practices (GMP), ISO standards, and Quality System Regulations (QSR). These regulations ensure compliance with quality and safety requirements for injectable medications.
Why are Good Manufacturing Practices (GMP) important?
GMP is crucial for reducing risks associated with particulate matter in injectable formulations, which can arise from excipients, active pharmaceutical ingredients (APIs), or the manufacturing process.
How do ISO standards contribute to parenteral injection safety?
ISO standards enhance safety compliance by ensuring that practices related to parenteral injection meet stringent quality benchmarks.
What is the role of Quality System Regulations (QSR)?
QSR provides a framework for maintaining quality throughout the manufacturing process, ensuring that all aspects of production meet regulatory standards.
How can organizations stay informed about regulatory updates?
Organizations should consistently review updates from oversight organizations such as the FDA and EMA to stay informed about evolving guidelines related to parenteral injections.
What impact can implementing a compliance checklist have on audit findings?
Implementing a compliance checklist can lead to significant improvements, as demonstrated by a pharmaceutical company that experienced a 30% reduction in audit findings.
What is the projected growth of the biologics sector?
The biologics sector is projected to have a compound annual growth rate of 15% through 2027.
What services does AVS Life Sciences provide?
AVS Life Sciences offers comprehensive GXP compliance services, including audits of API and Drug Product CMOs, Contract Test Labs, and other manufacturing and testing facilities, focusing on quality assurance and regulatory compliance.
