4 Key Practices for Effective Drug Label Compliance
Overview
Effective drug label compliance hinges on understanding the essential components of drug labels, navigating regulatory requirements, implementing thorough documentation and audit practices, and adopting continuous improvement strategies. These practices are not merely recommendations; they are critical for ensuring patient safety and meeting FDA standards. The significant number of drug recalls underscores the urgency for organizations to adapt to evolving regulations and maintain accurate labeling. By prioritizing these compliance practices, organizations can not only mitigate risks but also enhance their operational integrity and foster trust with stakeholders.
Introduction
In the intricate realm of pharmaceuticals, drug labels function as vital lifelines, ensuring that critical information about medications is effectively communicated to both healthcare providers and patients. The stakes are substantial; improper labeling can result in severe repercussions, including product recalls and adverse drug reactions that impact millions.
This article explores four essential practices that organizations can implement to bolster drug label compliance. It emphasizes the necessity of:
- Comprehending regulatory requirements
- Establishing robust documentation processes
- Cultivating a culture of continuous improvement
How can companies adeptly navigate the complex landscape of drug labeling while prioritizing patient safety and circumventing costly compliance missteps?
Understand Essential Components of Drug Labels
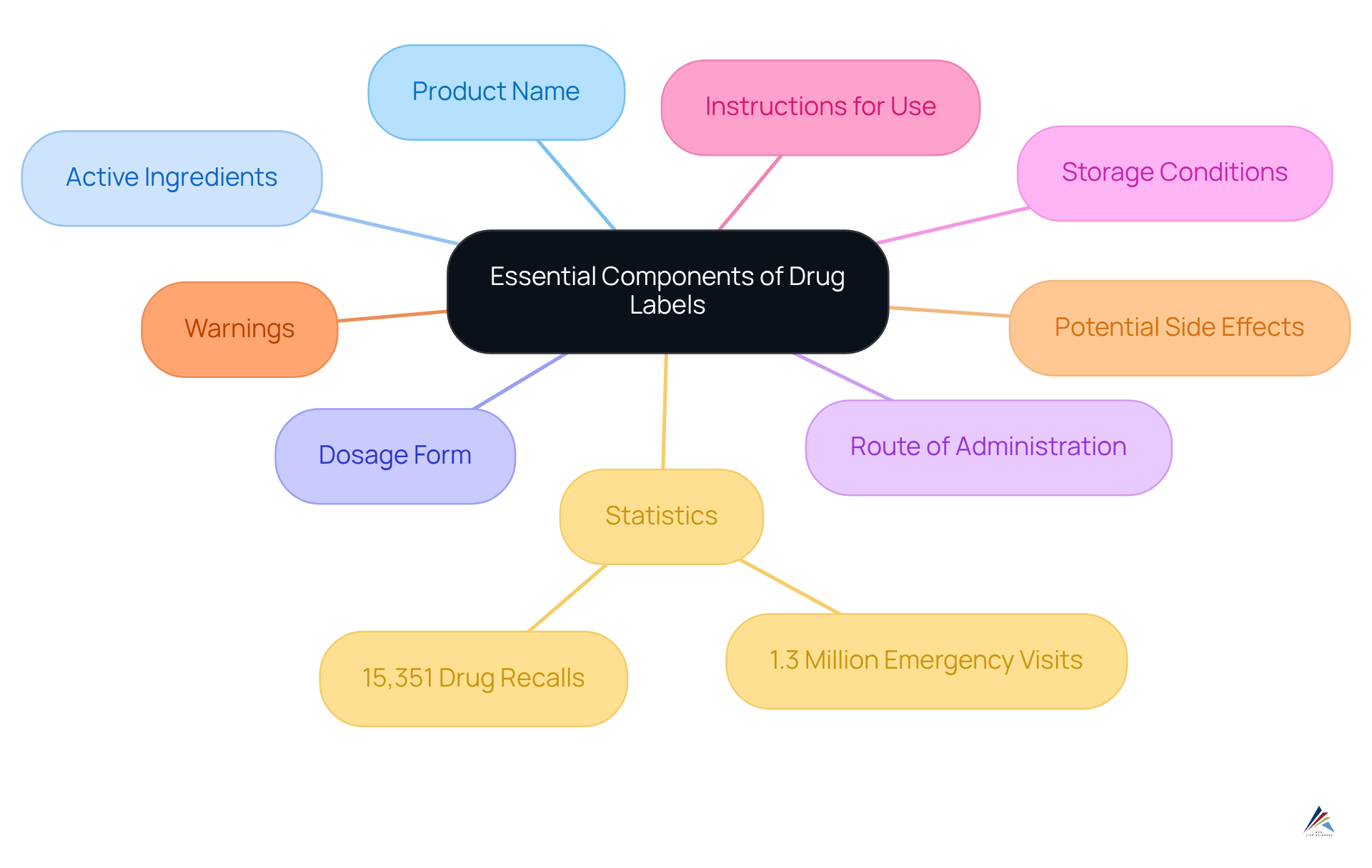
Drug labels are essential documents that must convey critical information, including the product name, active ingredients, dosage form, route of administration, and storage conditions. Furthermore, they must clearly outline the instructions for use, warnings, and potential side effects on the drug label. Adhering to these elements is crucial for meeting the standards established by the FDA as outlined in the drug label.
Since 2012, the FDA has issued over 15,351 drug recalls, which account for 94% of all FDA drug recalls, underscoring the importance of precise drug label information in avoiding compliance penalties and ensuring patient safety. A well-structured drug label not only enhances patient understanding but also reduces the risks associated with adverse drug reactions, which contribute to approximately 1.3 million emergency department visits annually, many of which result in serious reports.
To maintain these standards and protect patient well-being, organizations should conduct regular training sessions, ensuring that all team members understand the and the associated legal framework. As the FDA states, "A recall is defined as a firm’s removal or correction of a distributed product that the FDA deems is in violation of its laws."
Additionally, organizations must navigate challenges related to Good Manufacturing Practices (GMP) and Quality System Regulations (QSR) to ensure comprehensive compliance.
Navigate Regulatory Requirements for Drug Labeling
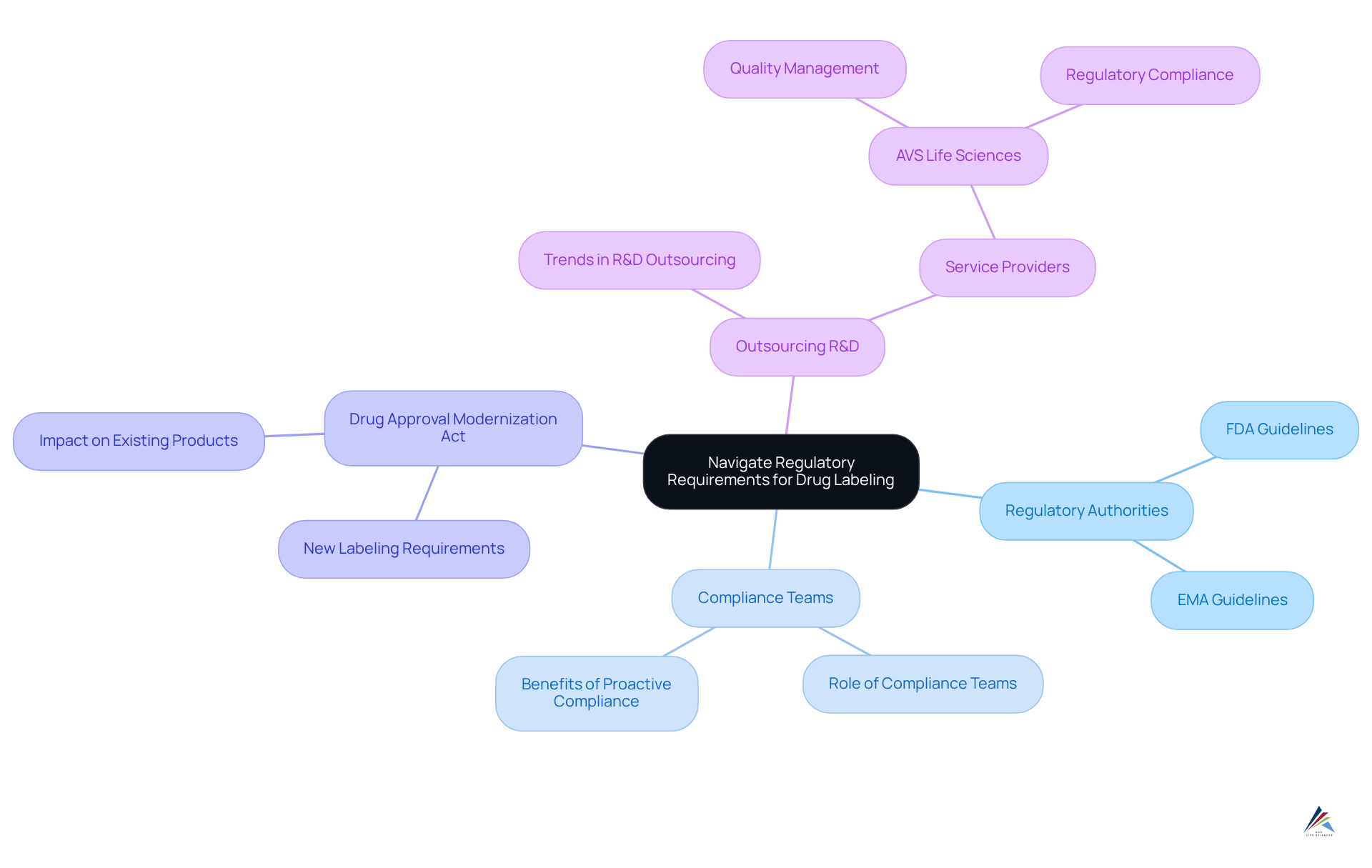
Organizations must remain vigilant regarding the evolving regulatory landscape, particularly the guidelines set forth by the FDA, EMA, and other pertinent authorities. Consistently examining updates to marking requirements is crucial for grasping the consequences of these modifications. The Drug Approval Modernization Act, for instance, has introduced significant new labeling requirements that demand a comprehensive understanding of their impact on existing products.
Creating a dedicated compliance team to oversee these regulations can significantly improve a company's ability to adapt, ensuring that all drug labels conform to current compliance standards. This proactive method not only reduces risks but also promotes a culture of quality and accountability within the entity.
As J.P. Garnier, Chief Executive of GlaxoSmithKline, noted, '[Pharma’s traditional strategy is a business model where you are guaranteed to lose your entire book of business every 10 to 12 years.]' This highlights the necessity for entities to continuously adapt to regulatory changes.
Moreover, statistics show that numerous pharmaceutical firms are progressively to improve efficiency and adherence, emphasizing the significance of a strong adherence strategy. By utilizing the knowledge of service providers such as AVS Life Sciences, organizations can manage these complexities effectively, ensuring adherence and maintaining high standards of quality.
Implement Effective Documentation and Audit Practices
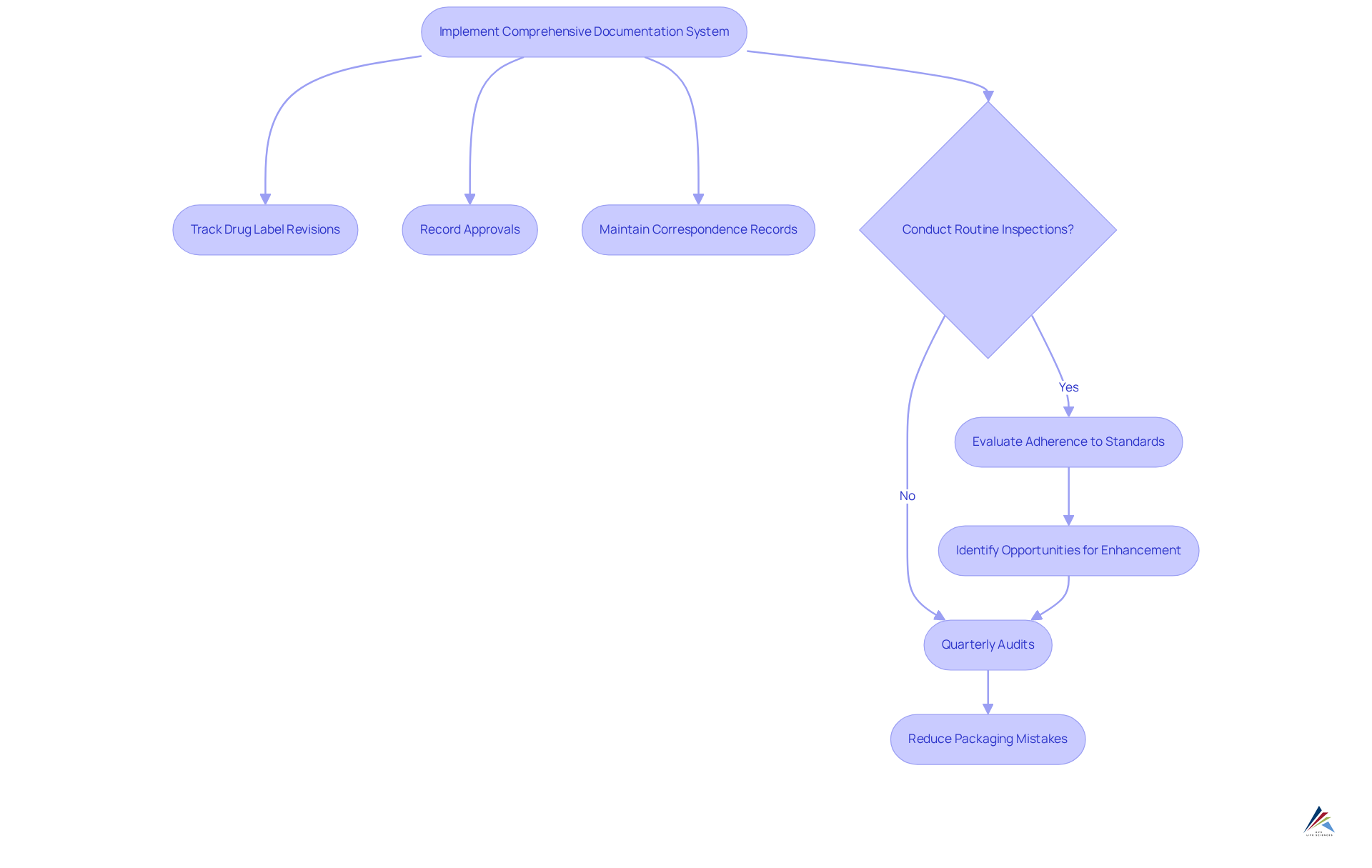
Implementing a comprehensive documentation system is essential for tracking changes to the drug label and ensuring that all information remains accurate. This system must include meticulous records of:
- Drug label revisions
- Approvals
- Correspondence with regulatory bodies
Routine inspections should be conducted to evaluate adherence to and identify opportunities for enhancement. For instance, a drug manufacturer that instituted quarterly audits found they could reduce packaging mistakes by 30%, significantly improving their regulatory standing.
Furthermore, utilizing software solutions for document management can streamline this process and enhance overall efficiency. By prioritizing these strategies, organizations can ensure compliance and bolster their operational effectiveness.
Adopt Continuous Improvement Strategies for Compliance
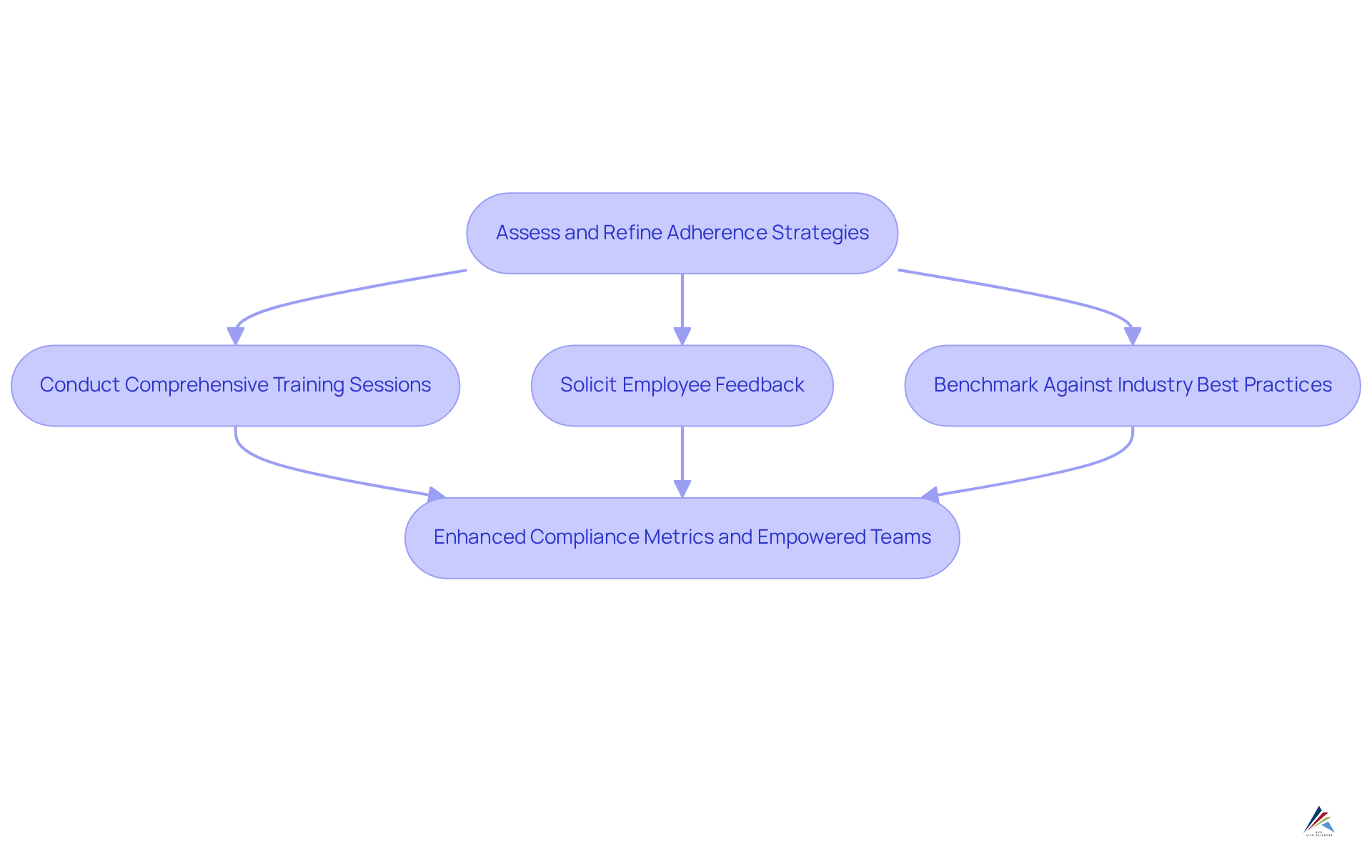
To foster a culture of , organizations must diligently assess and refine their adherence strategies. This involves:
- Conducting comprehensive training sessions
- Actively soliciting employee feedback
- Benchmarking against industry best practices
For instance, a company that implemented an ongoing feedback system with its tagging team effectively identified and addressed regulatory issues proactively, leading to a more robust tagging process. Furthermore, the integration of regulatory management software can streamline the ongoing oversight and enhancement of tagging practices, ensuring that organizations adapt swiftly to the evolving regulatory landscape. Such proactive measures not only enhance compliance metrics but also empower teams to contribute to a more efficient and compliant drug label process.
As Benjamin Franklin wisely stated, "Without continual growth and progress, such words as improvement, achievement, and success have no meaning.
Conclusion
Effective drug label compliance is paramount for ensuring patient safety and maintaining regulatory standards. Understanding the essential components of drug labels allows organizations to significantly reduce the risk of recalls and adverse reactions. This commitment to precise labeling not only fulfills FDA requirements but also fosters a culture of accountability and quality within the pharmaceutical industry.
The article outlines four key practices:
- Understanding the components of drug labels
- Navigating regulatory requirements
- Implementing effective documentation and audit practices
- Adopting continuous improvement strategies
Each of these practices plays a critical role in enhancing compliance, from establishing a thorough understanding of labeling requirements to fostering a proactive approach that anticipates regulatory changes and promotes operational efficiency.
Ultimately, the importance of maintaining rigorous drug label compliance cannot be overstated. Organizations must prioritize these practices to safeguard patient health and navigate the complexities of the regulatory landscape. By doing so, they protect their reputation and contribute to the broader mission of delivering safe and effective medications to the public. Embracing these best practices today will ensure a more compliant and successful future in pharmaceutical labeling.
Frequently Asked Questions
What are the essential components of drug labels?
Drug labels must convey critical information, including the product name, active ingredients, dosage form, route of administration, storage conditions, instructions for use, warnings, and potential side effects.
Why is precise drug label information important?
Precise drug label information is crucial for avoiding compliance penalties, ensuring patient safety, and reducing the risks associated with adverse drug reactions, which contribute to approximately 1.3 million emergency department visits annually.
How many drug recalls has the FDA issued since 2012?
Since 2012, the FDA has issued over 15,351 drug recalls, which account for 94% of all FDA drug recalls.
What role do training sessions play in maintaining drug label standards?
Regular training sessions help ensure that all team members understand the importance of drug label elements and the associated legal framework, which is essential for maintaining compliance and protecting patient well-being.
What are Good Manufacturing Practices (GMP) and Quality System Regulations (QSR)?
GMP and QSR are regulations that organizations must navigate to ensure comprehensive compliance with drug labeling and manufacturing standards.
