4 Key Practices for Effective Dietary Supplement Manufacturing

Overview
The four key practices for effective dietary supplement manufacturing are essential for overcoming compliance challenges and ensuring product excellence.
- Establishing quality management systems is crucial; these systems form the backbone of regulatory adherence and operational efficiency.
- Strict adherence to regulatory standards and good manufacturing practices guarantees that products meet industry requirements and consumer safety expectations.
- Implementing robust documentation and record-keeping practices not only supports compliance but also enhances traceability and accountability in operations.
- Providing ongoing training and development for manufacturing personnel fosters a culture of continuous improvement and expertise within the workforce.
Together, these practices ensure compliance with regulations, enhance product quality, and promote an environment of continuous improvement, as highlighted by the article's emphasis on structured processes, employee education, and systematic documentation to maintain high standards in the manufacturing industry.
Introduction
The dietary supplement industry faces heightened scrutiny as consumers increasingly demand superior quality and safety standards. In order to thrive within this competitive landscape, manufacturers must adeptly navigate a complex web of regulations and best practices. This article delves into four essential practices that not only ensure compliance but also bolster operational efficiency and product integrity.
How can manufacturers effectively implement these strategies to satisfy both regulatory requirements and consumer expectations while cultivating a culture of continuous improvement?
Establish Quality Management Systems for Dietary Supplements
To establish an effective Quality Management System (QMS) for dietary supplements, organizations must focus on key components, leveraging the expertise of AVS Life Sciences.
-
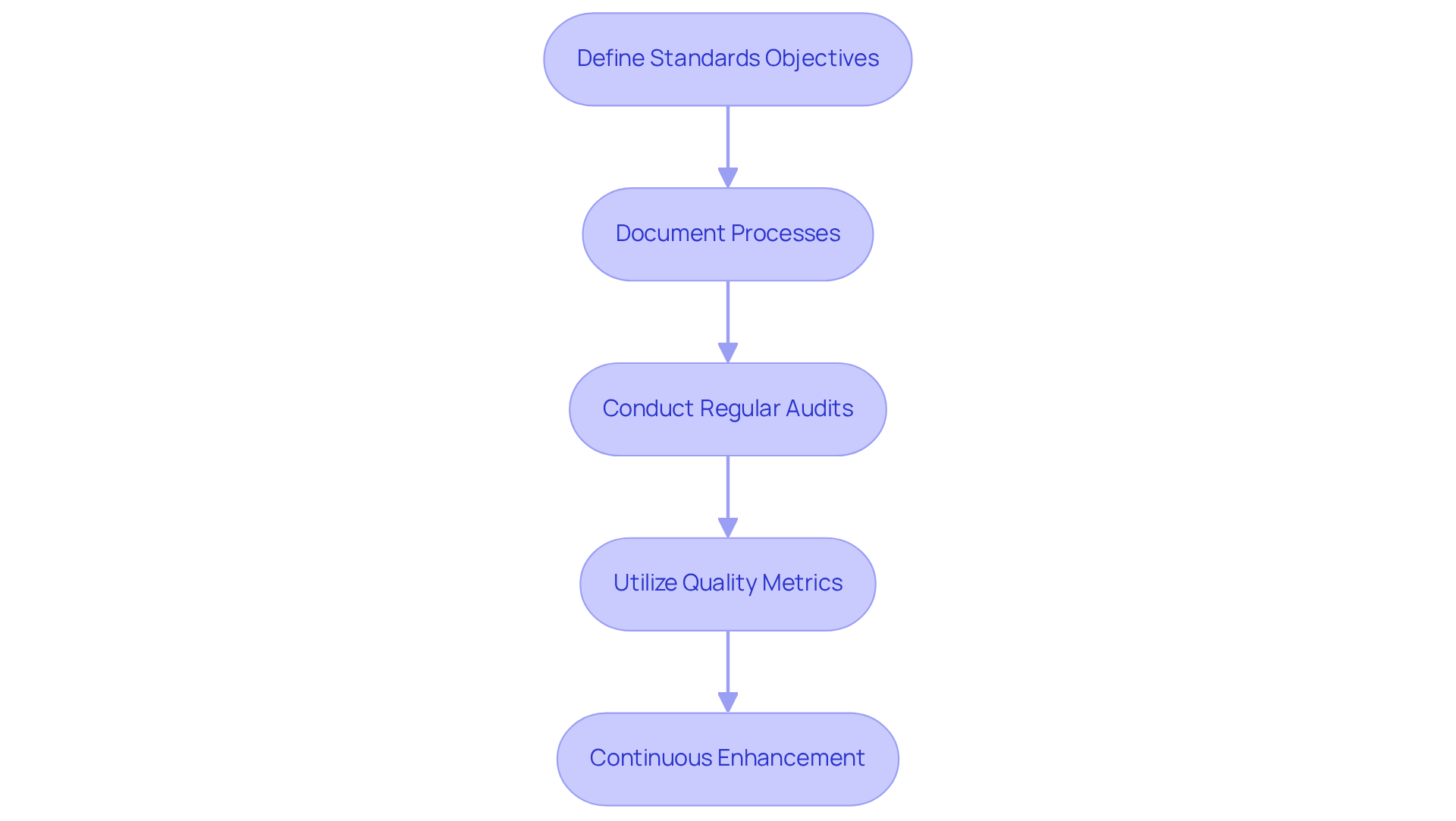
Define Standards Objectives: Clearly outline performance goals that align with regulatory requirements and customer expectations. This involves establishing measurable goals for product excellence and safety, ensuring adherence to FDA regulations and GXP standards. By doing so, organizations can set a solid foundation for their QMS.
-
Document Processes: Create detailed documentation for all processes involved in dietary supplement manufacturing. This encompasses Standard Operating Procedures (SOPs), work instructions, and manuals that direct employees in upholding standards, reflecting AVS Life Sciences' dedication to excellent documentation practices in dietary supplement manufacturing. Additionally, user guides can aid in the training and onboarding of personnel, ensuring consistency in management practices.
-
Conduct Regular Audits: Establish a timetable for internal evaluations to examine adherence to set performance standards. This proactive approach helps identify areas for improvement and ensures that the QMS is functioning effectively, in line with AVS Life Sciences' internal and external auditing techniques. Case studies showcasing successful audit outcomes can provide valuable insights into best practices, reinforcing the importance of regular assessments.
-
Utilize Quality Metrics: Establish key performance indicators (KPIs) to monitor the effectiveness of the QMS. Metrics such as defect rates, customer complaints, and audit findings can provide insights into the system's performance, supporting a culture of continuous improvement. By regularly reviewing these metrics, organizations can make informed decisions to enhance their QMS.
-
Continuous Enhancement: Promote a culture of ongoing enhancement by frequently evaluating and revising the QMS based on audit results, employee input, and changes in compliance requirements. This proactive method guarantees that the QMS remains pertinent and efficient, embodying the principles of management excellence and adherence to regulations that AVS Life Sciences advocates. As organizations strive for compliance, they must embrace a mindset of continuous improvement.
Adhere to Regulatory Standards and Good Manufacturing Practices
To ensure compliance with regulatory standards and Good Manufacturing Practices (GMP), manufacturers must adopt essential practices that address compliance challenges effectively:
-
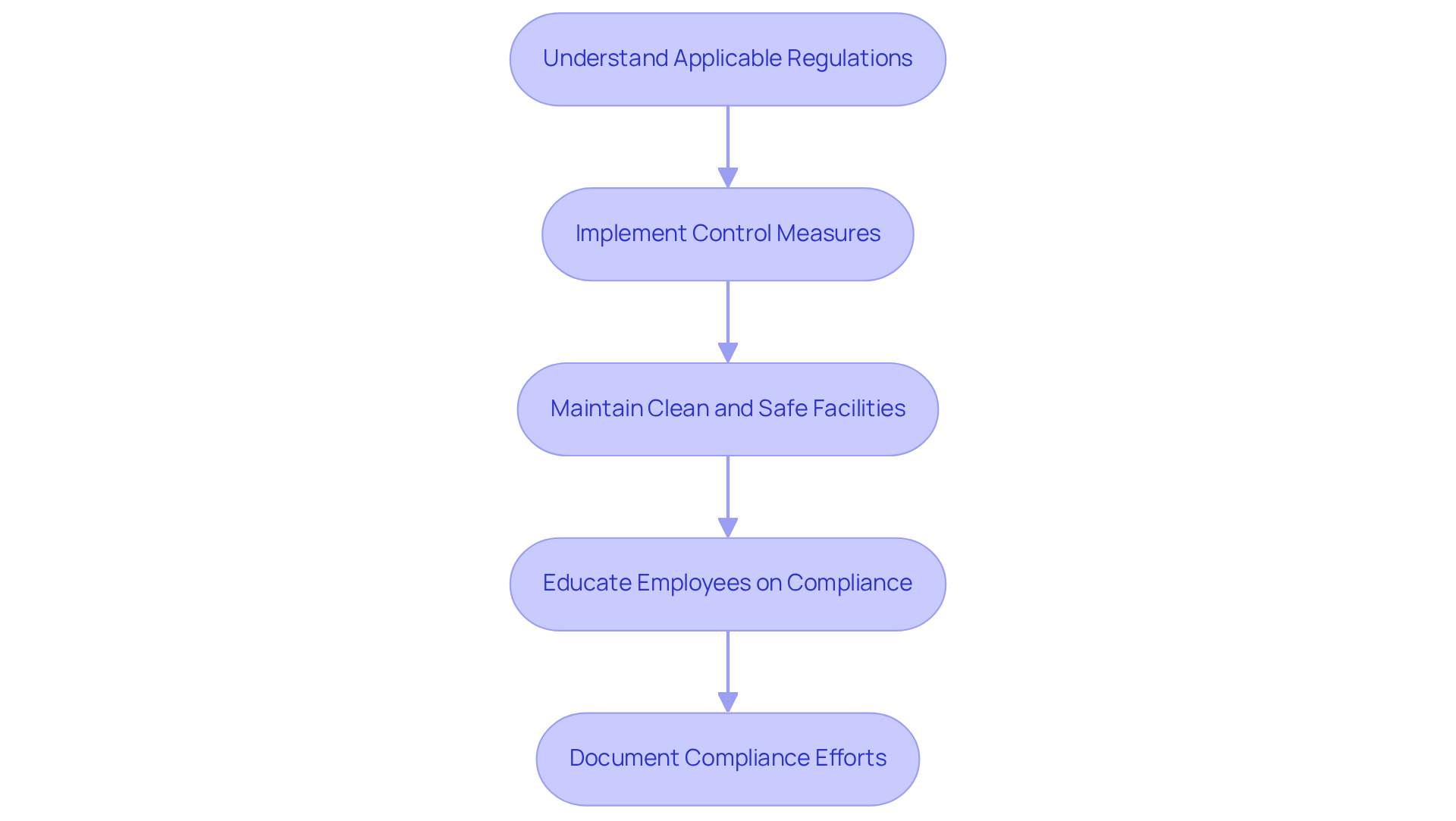
Understand Applicable Regulations: Familiarizing oneself with the FDA's Current Good Manufacturing Practices (cGMPs) for dietary supplements is crucial. This encompasses comprehending the requirements for dietary supplement manufacturing, as well as for packaging, labeling, and storage. Adherence to these regulations is not merely best practice; it is a legal obligation in many regions, including the United States.
-
Implement Control Measures: Establishing rigorous control protocols is vital. This should include ingredient testing, batch testing, and final product testing to ensure that all products consistently meet safety and performance standards before they reach consumers. Effective control measures significantly decrease the risk of contamination and flaws, thereby boosting consumer trust. Collaborating with AVS Life Sciences can provide professional consulting to create and execute these control measures effectively, including validation engineering support and regulatory submissions guidance.
-
Maintain Clean and Safe Facilities: Manufacturing facilities must be designed and maintained according to GMP standards. This includes implementing proper sanitation practices, pest control measures, and employee hygiene protocols to prevent contamination. A well-maintained facility not only adheres to regulations but also enhances the overall standard of the products produced. AVS Life Sciences focuses on converting GMP facilities to guarantee adherence and excellence for biotechnology clients.
-
Educate Employees on Compliance: Comprehensive instruction for all employees regarding regulatory requirements and GMP is essential. This ensures that all participants in the manufacturing process understand their duties in upholding standards and quality. Frequent updates are necessary to keep staff informed about any changes in regulations or manufacturing processes. AVS Life Sciences provides customized consulting solutions to enhance employee development initiatives, ensuring that your team is well-prepared to meet regulatory standards.
-
Document Compliance Efforts: Keeping comprehensive records of all compliance-related activities, including training sessions, audits, and control tests, is essential. This documentation serves as proof of conformity during inspections and audits, showcasing a commitment to quality and adherence to rules. AVS Life Sciences can assist in developing robust documentation systems to streamline this process.
By implementing these practices, manufacturers not only meet regulatory requirements but also enhance their reputation and marketability in the competitive dietary supplement manufacturing industry. It is significant to note that cGMP certification typically endures for one to three years, necessitating continuous adherence and regular audits. Many retailers and distributors prefer cGMP-certified products, which can significantly expand market reach. Producers should remain vigilant against common traps in the adherence process, such as insufficient training or inadequate documentation, which can lead to costly errors. Specific instances of these traps include failing to stay updated with regulatory changes or neglecting to adequately document control procedures.
Implement Robust Documentation and Record-Keeping Practices
To implement robust documentation and record-keeping practices, manufacturers must consider the following key strategies:
-
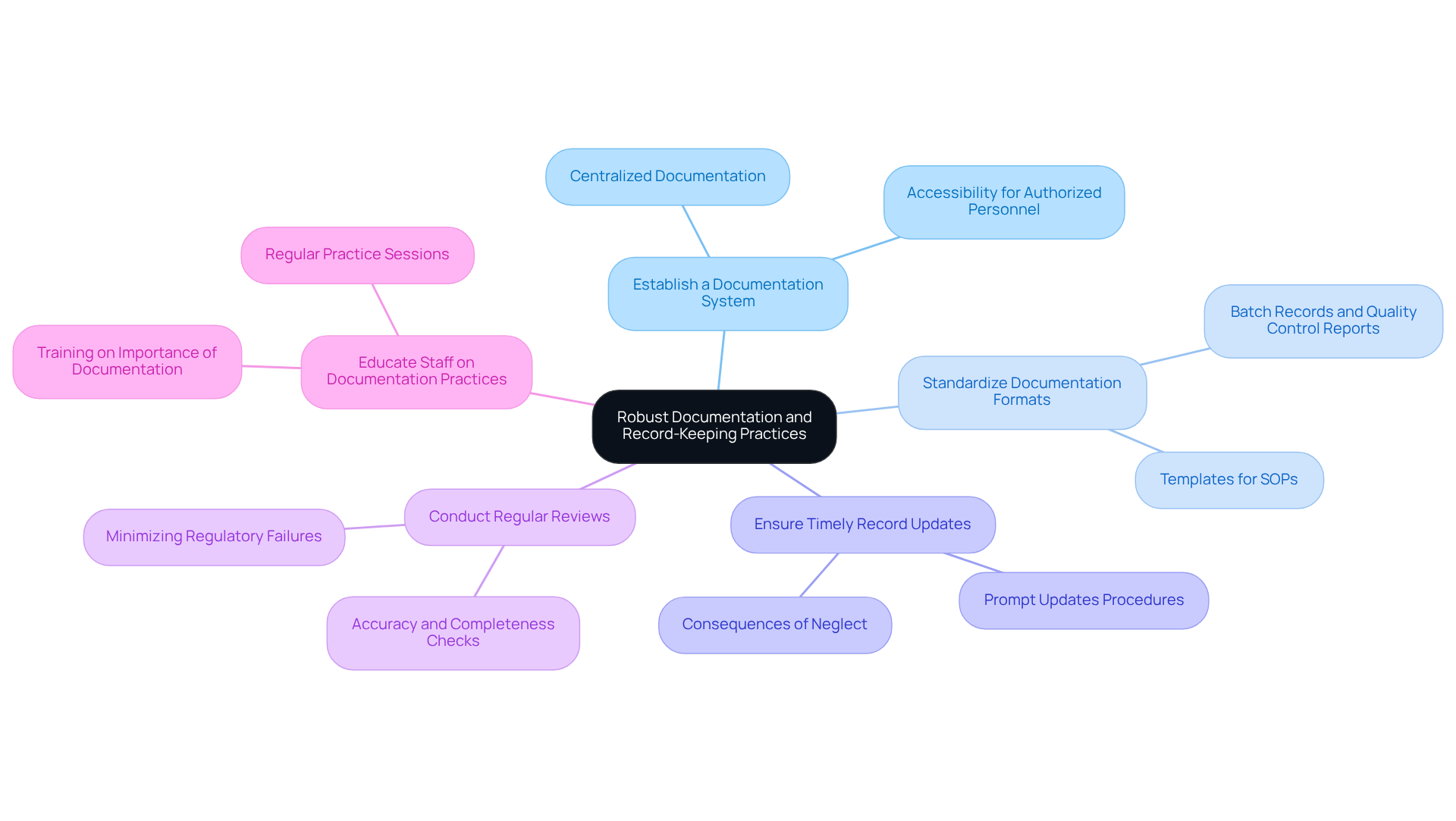
Establish a Documentation System: Create a centralized documentation system that encompasses all critical records related to manufacturing processes, control of standards, and regulatory activities. This system should be easily accessible to authorized personnel, facilitating efficient information retrieval and management.
-
Standardize Documentation Formats: Utilize standardized formats for all documentation to ensure consistency and clarity across the organization. This includes templates for Standard Operating Procedures (SOPs), batch records, and quality control reports, which assist in reducing errors and improving adherence to Good Manufacturing Practices (GMP).
-
Ensure Timely Record Updates: Implement procedures for prompt updates to records, ensuring that all documentation reflects the most current practices and regulatory requirements. Organizations that neglect proper documentation can face significant penalties, including hefty fines and sanctions.
-
Conduct Regular Reviews: Schedule regular reviews of documentation to ensure accuracy and completeness. This proactive strategy aids in recognizing gaps or inconsistencies that require attention, thus minimizing the risk of regulatory failures. Notably, an increase in FDA observations related to dietary supplements underscores the critical nature of maintaining rigorous documentation practices.
-
Educate Staff on Documentation Practices: Provide thorough instruction for employees on the significance of precise documentation and the specific procedures for record-keeping. This ensures that all team members understand their responsibilities in upholding regulations, fostering a culture of accountability and ongoing improvement. Regular practice sessions can reinforce best practices and help mitigate human errors, such as typos and inaccurate data entries, which frequently lead to documentation issues.
By adopting these practices, dietary supplement manufacturing companies can enhance their preparedness and operational efficiency, ultimately safeguarding their businesses against legal penalties and reputational harm.
Provide Ongoing Training and Development for Manufacturing Personnel
To ensure ongoing training and development for manufacturing personnel in dietary supplement manufacturing, organizations must adopt essential practices that address compliance challenges and enhance workforce capabilities.
-
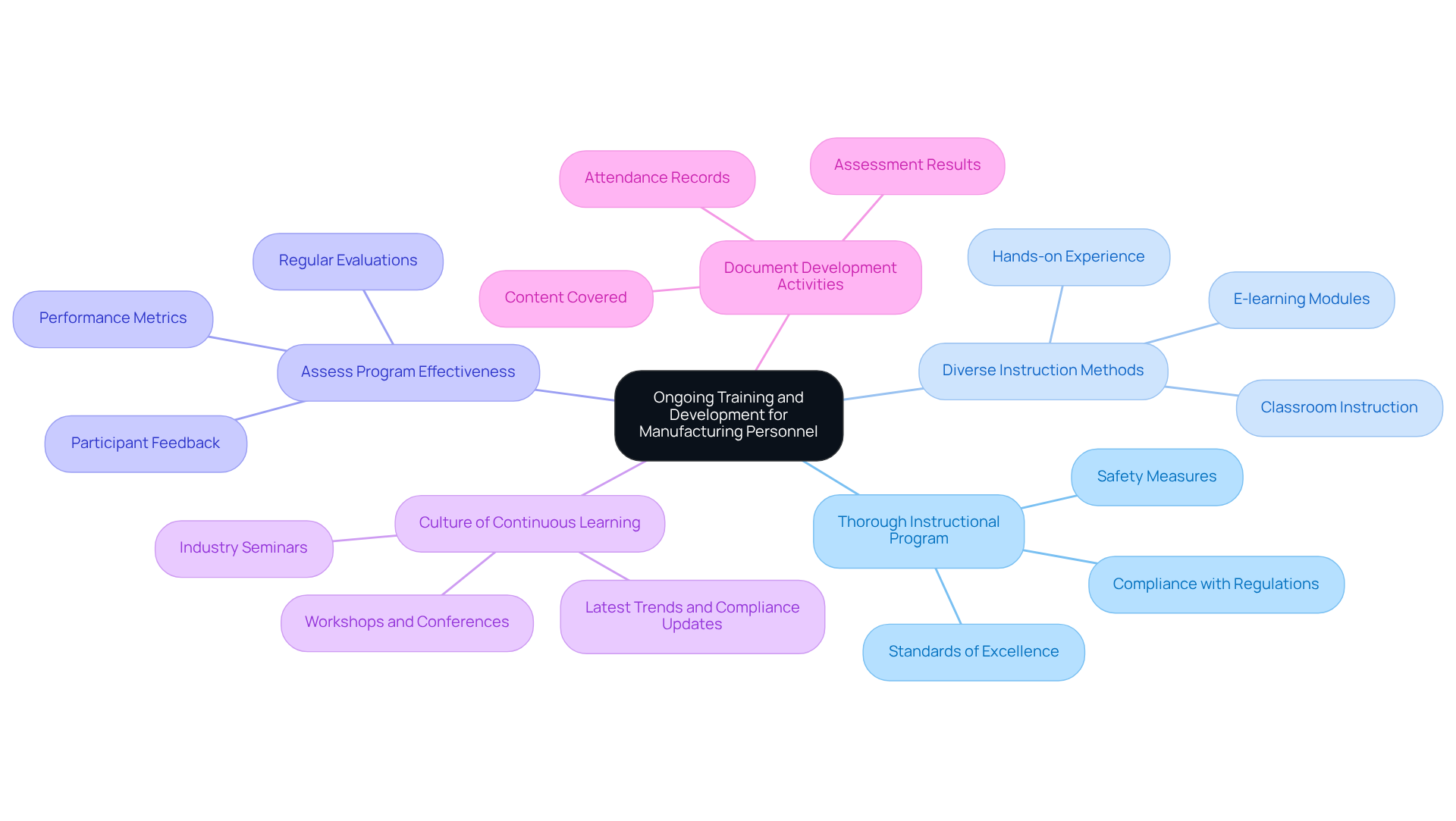
Create a Thorough Instructional Program: Establish a robust instructional program encompassing all facets of dietary supplement production, including compliance with regulations, standards of excellence, and safety measures. This foundational step is critical for equipping staff with the necessary knowledge and skills, aligning with AVS Life Sciences' commitment to delivering comprehensive regulatory and quality solutions across biopharmaceuticals, medical devices, and dietary supplement manufacturing.
-
Utilize Diverse Instruction Methods: Implement a variety of educational approaches, such as classroom instruction, hands-on experience, and e-learning modules. This diversity caters to different learning preferences and enhances engagement, making the educational experience more effective. AVS Life Sciences can assist organizations in developing these methods to ensure optimal learning outcomes.
-
Assess Program Effectiveness: Regularly evaluate the impact of development initiatives through assessments, participant feedback, and performance metrics. Continuous assessment aids in identifying areas for enhancement and ensures that training in dietary supplement manufacturing aligns with industry standards and compliance needs—a principle that AVS Life Sciences emphasizes in its consulting services.
-
Encourage a Culture of Continuous Learning: Foster an environment that values continuous learning by providing employees with opportunities to engage in workshops, conferences, and industry seminars. Keeping staff informed about the latest trends and compliance updates is vital for maintaining adherence and promoting professional growth. AVS Life Sciences distinguishes itself as a reliable ally in dietary supplement manufacturing, providing expert consulting for validation and compliance strategies in the nutraceutical sector.
-
Document Development Activities: Maintain comprehensive records of all development activities, including attendance, content covered, and assessment results. This documentation is essential for demonstrating compliance during audits and inspections, ensuring organizations can effectively showcase their commitment to quality and regulatory adherence—a key focus of AVS Life Sciences.
Incorporating these practices not only enhances the skill set of personnel in dietary supplement manufacturing but also aligns with the industry's evolving standards. As highlighted by the recent statistic that manufacturing workers' wages in Montana increased by an average of $8,000, investing in skill development can yield significant economic benefits. Furthermore, as Ravinder Tulsiani noted, "Training sessions are vital to the learning process, but they are only one step in the learning process and this should never be forgotten." This underscores the importance of continuous education in the dietary supplement sector. Additionally, AVS Life Sciences, with its 80% repeat business rate, exemplifies the effectiveness of comprehensive training programs in ensuring quality management and regulatory compliance.
Conclusion
Establishing effective dietary supplement manufacturing practices is essential for ensuring product quality, regulatory compliance, and operational excellence. By focusing on quality management systems, adhering to regulatory standards, implementing robust documentation practices, and providing ongoing training for personnel, manufacturers can significantly enhance their processes and outcomes in the competitive supplement market.
The importance of defining clear standards, conducting regular audits, and utilizing quality metrics cannot be overstated. These practices foster a culture of continuous improvement. Additionally, understanding and implementing Good Manufacturing Practices (GMP) not only fulfills legal obligations but also builds consumer trust through enhanced product safety. Furthermore, robust documentation and effective training programs are critical for maintaining compliance and empowering employees to uphold the highest manufacturing standards.
Ultimately, the practices outlined in this article serve as a roadmap for dietary supplement manufacturers aiming to elevate their operations. Embracing these strategies mitigates risks associated with regulatory failures while positioning companies for success in a rapidly evolving industry. By prioritizing quality and compliance, manufacturers can ensure their products meet consumer expectations, fostering a culture of excellence that drives long-term growth and sustainability.
Frequently Asked Questions
What is a Quality Management System (QMS) for dietary supplements?
A Quality Management System (QMS) for dietary supplements is a structured framework that organizations implement to ensure product excellence and safety, aligning with regulatory requirements and customer expectations.
How can organizations define standards objectives for their QMS?
Organizations can define standards objectives by clearly outlining performance goals that align with regulatory requirements and customer expectations, establishing measurable goals for product excellence and safety in compliance with FDA regulations and GXP standards.
What documentation is necessary for dietary supplement manufacturing?
Necessary documentation includes Standard Operating Procedures (SOPs), work instructions, and manuals that guide employees in maintaining standards, as well as user guides to assist in training and onboarding personnel.
Why are regular audits important for a QMS?
Regular audits are important as they help organizations evaluate adherence to performance standards, identify areas for improvement, and ensure the QMS is functioning effectively, promoting best practices and continuous enhancement.
What are quality metrics, and why are they used in a QMS?
Quality metrics are key performance indicators (KPIs) used to monitor the effectiveness of the QMS. Metrics such as defect rates, customer complaints, and audit findings provide insights into system performance and support a culture of continuous improvement.
How can organizations promote continuous enhancement of their QMS?
Organizations can promote continuous enhancement by frequently evaluating and revising the QMS based on audit results, employee input, and changes in compliance requirements, ensuring the system remains relevant and efficient.
