4 Key Practices for Cloud-Based Validation Solutions

Overview
This article highlights four critical practices for implementing effective cloud-based validation solutions within the life sciences sector:
- Conducting thorough risk assessments is essential to identify potential vulnerabilities.
- Establishing clear documentation ensures that all processes are transparent and compliant with regulatory standards.
- Utilizing automated tools streamlines validation efforts and enhances accuracy.
- Ensuring continuous training and support for compliance teams is vital for maintaining adherence to evolving regulations.
Together, these practices significantly enhance data security, regulatory compliance, and operational efficiency in cloud environments.
Introduction
Cloud-based validation solutions are revolutionizing the life sciences sector by delivering unparalleled data accessibility and ensuring compliance with rigorous regulations. As organizations endeavor to adapt to shifting standards, the adoption of these technologies presents substantial advantages, including:
- Improved collaboration
- Optimized processes
Nonetheless, the shift to cloud-based systems is not without its challenges, notably:
- Data security concerns
- Integration complexities
How can companies effectively navigate these obstacles to fully capitalize on the advantages of cloud validation?
Understand the Importance of Cloud-Based Validation in Life Sciences
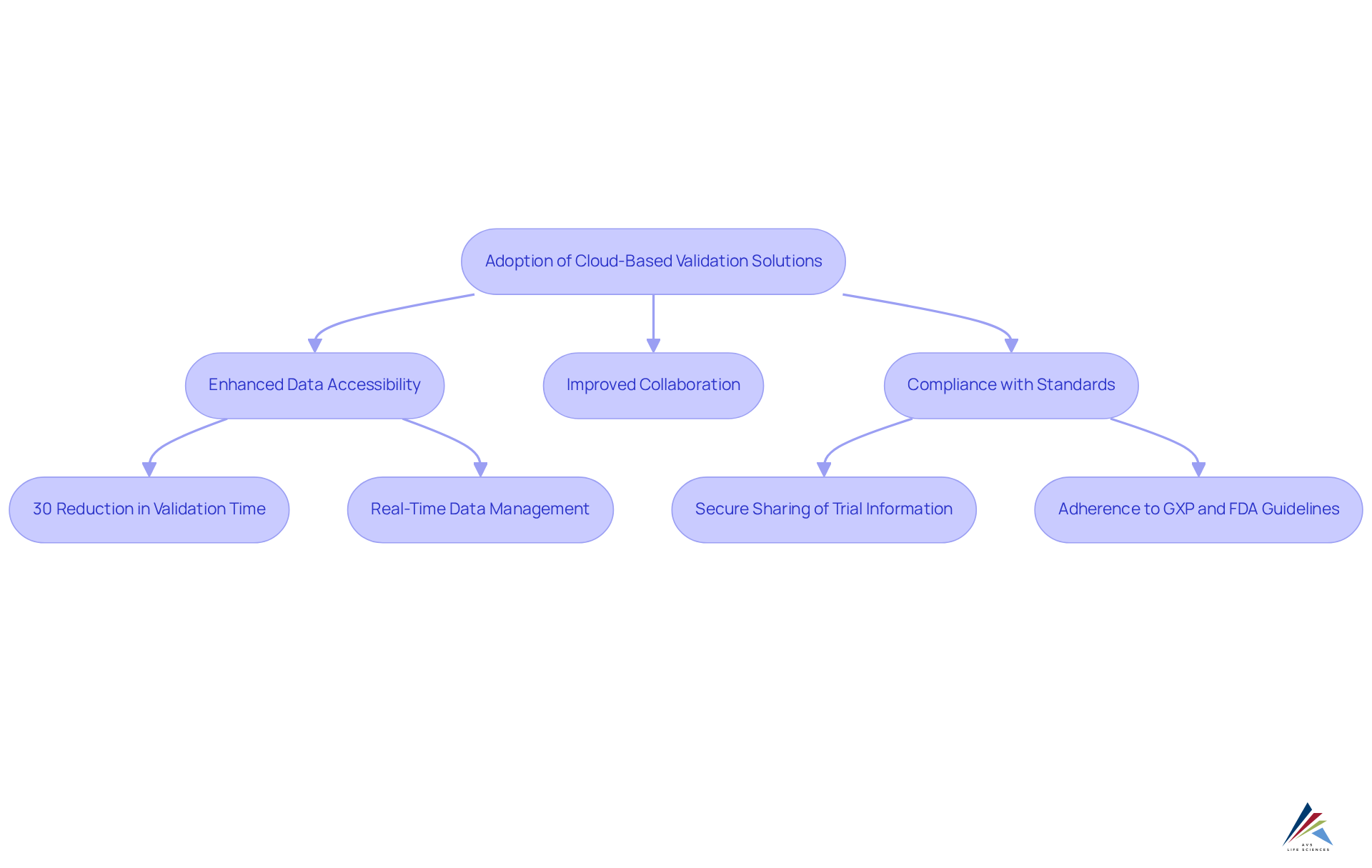
Cloud-based validation solutions are increasingly vital in the life sciences sector, significantly enhancing data accessibility, collaboration, and compliance with standards such as GXP and FDA guidelines. By leveraging cloud technology, organizations achieve real-time data management, which is critical for maintaining the integrity of verification processes. These solutions streamline updates and offer scalability, enabling companies to swiftly adapt to evolving compliance requirements.
For instance, a pharmaceutical firm that implemented cloud-based validation solutions realized a remarkable 30% reduction in validation time, expediting product launches while ensuring adherence to Good Manufacturing Practices (GMP). Furthermore, cloud platforms empower pharmaceutical companies to securely share anonymized trial information with oversight organizations, thereby enhancing transparency and compliance.
AVS Life Sciences underscores the significance of Standard Operating Procedures (SOPs) and robust documentation practices in their quality management solutions, ensuring that organizations not only meet but surpass industry standards. Through expert consulting, AVS Life Sciences provides tailored methodologies and services that elevate compliance and quality management in the life sciences domain.
Identify Key Challenges in Transitioning to Cloud-Based Validation
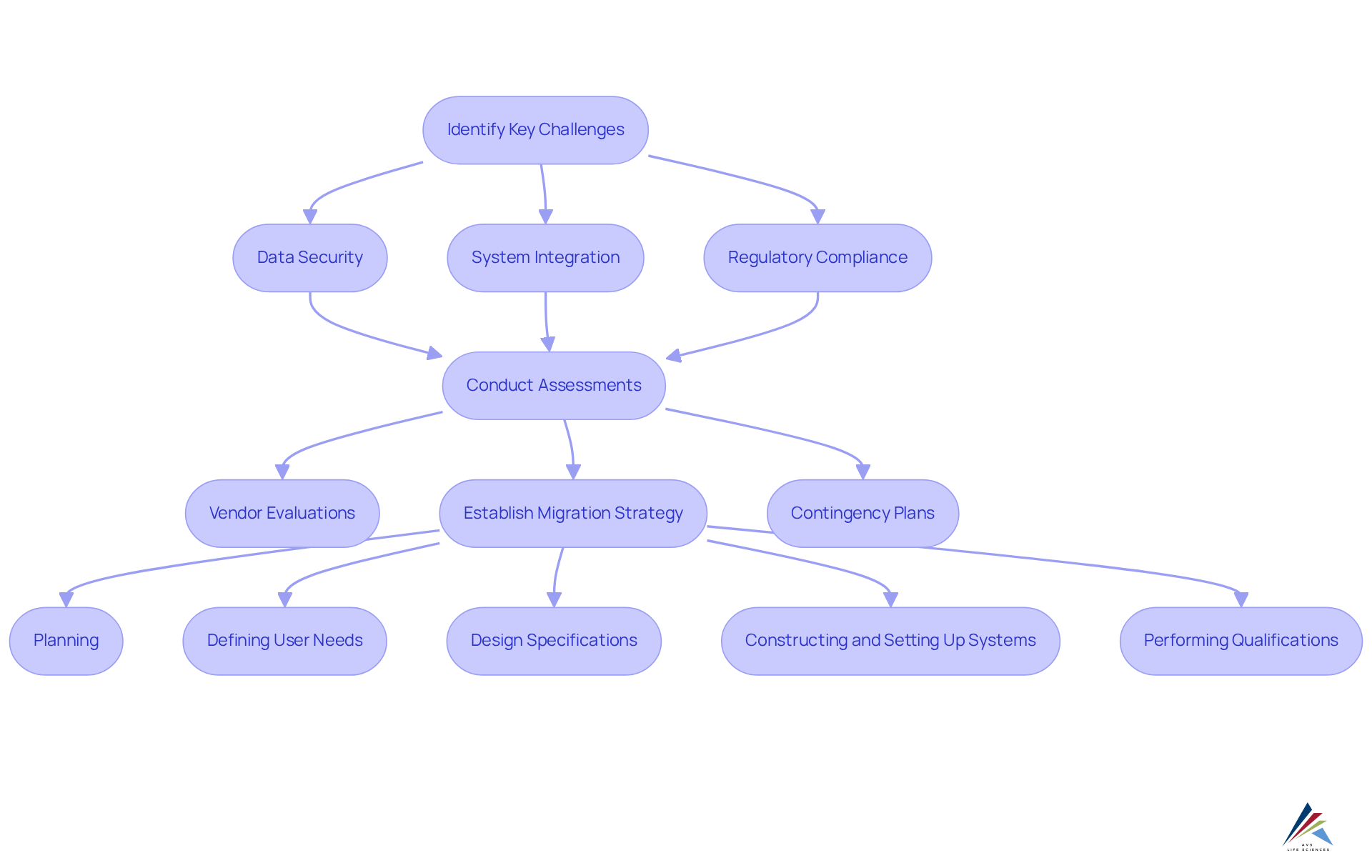
Switching to cloud-based validation solutions poses significant challenges for biotech companies, particularly in the realms of data security, integration with existing systems, and compliance with regulatory requirements. Data security issues are paramount, as sensitive information must be safeguarded against breaches during the migration process.
For example, a biotech company faced considerable obstacles when attempting to integrate its legacy systems with a new cloud validation platform, leading to project delays and increased costs.
To effectively tackle these challenges, organizations should:
- Conduct comprehensive assessments of their current systems
- Prioritize rigorous vendor evaluations
- Establish a clear migration strategy that includes contingency plans for potential setbacks
This proactive approach not only protects data integrity but also ensures adherence to industry regulations, ultimately facilitating a smoother transition to cloud-based validation solutions.
By following the stages of CSV—planning, defining user needs, design specifications, constructing and setting up systems, and performing installation, operational, and performance qualifications—biotech companies can enhance their verification processes and ensure that their cloud-based validation solutions meet industry standards.
AVS Life Sciences stands ready to assist entities in adopting these best practices, ensuring quality management and compliance during the transition.
Implement Best Practices for Cloud-Based Validation Solutions
To effectively implement cloud-based validation solutions, organizations must adopt several best practices:
-
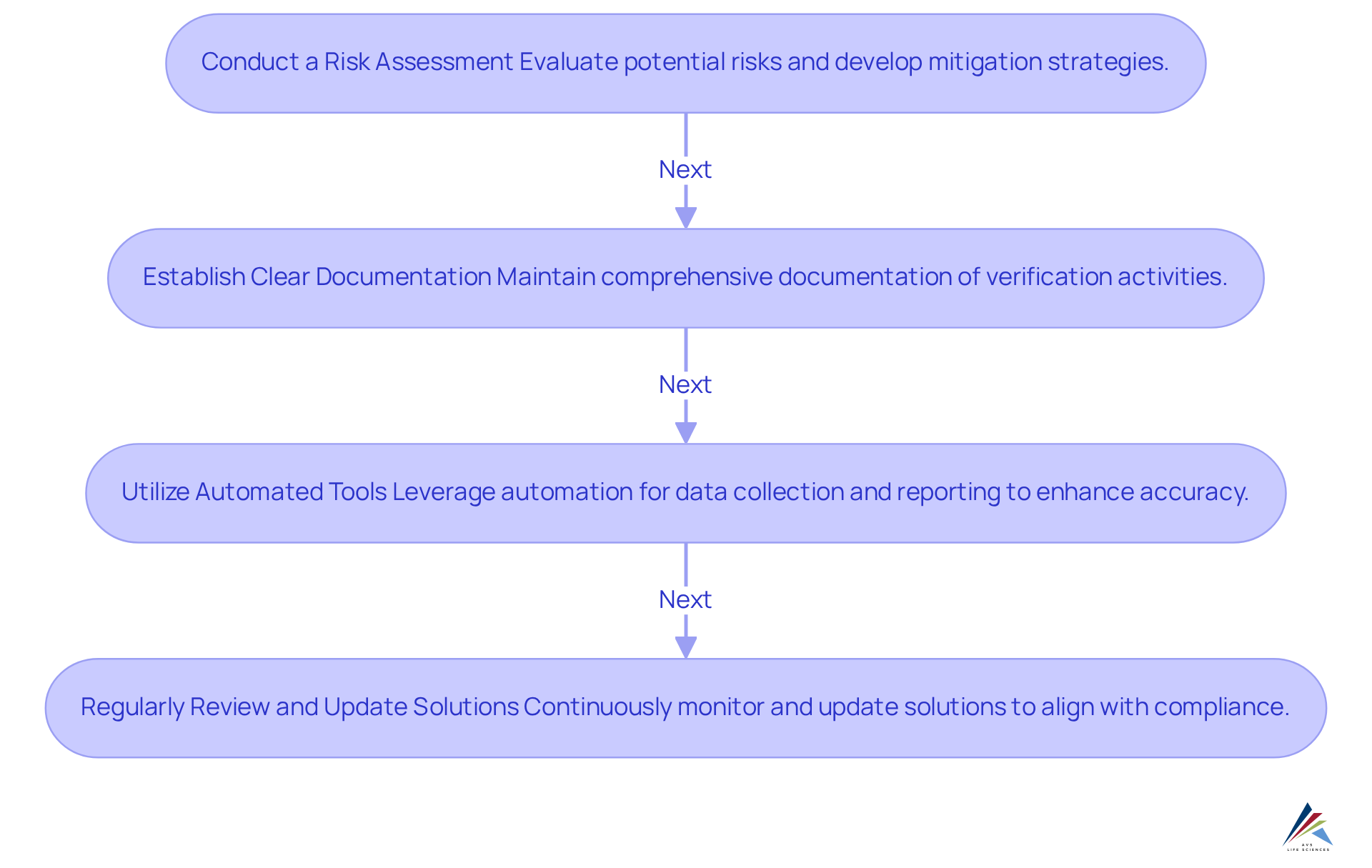
Conduct a Risk Assessment: Evaluating potential risks associated with cloud storage and processing is essential. This assessment identifies vulnerabilities and develops appropriate mitigation strategies, ensuring sensitive data remains secure and compliant with industry standards. The critical need for robust security measures is underscored by incidents such as the 2014 cyberattack on JPMorgan Chase, which compromised sensitive information from over 80 million households. Additionally, aligning risk assessments with GXP standards and FDA regulations ensures organizations meet compliance requirements effectively.
-
Establish Clear Documentation: Maintaining comprehensive documentation of all verification activities is crucial. This includes protocols, results, and any deviations encountered during the verification process. Comprehensive documentation aids adherence to regulatory standards and enables audits and inspections, thereby strengthening the integrity of the verification process. Implementing Standard Operating Procedures (SOPs) can enhance documentation practices further, ensuring consistency and adherence to Good Manufacturing Practices (GMP). The absence of multi-factor authentication (MFA) allowed attackers to exploit vulnerabilities, emphasizing the need for meticulous documentation in security practices, as noted by Change Healthcare.
-
Utilize Automated Tools: Leveraging automation for data collection and reporting significantly enhances accuracy and reduces the likelihood of human error. Automated tools simplify the verification process, ensuring data integrity is preserved and compliance requirements are fulfilled efficiently. This method conforms to the standards for Computer System Verification (CSV), offering companies expert assistance from AVS Life Sciences to enhance their verification efforts.
-
Regularly reviewing and updating cloud-based validation solutions is essential for continuous monitoring to align with changing compliance requirements and technological advancements. This proactive strategy helps entities stay ahead of regulatory challenges and adapt to shifts in the legal landscape. The ransomware attack on Change Healthcare in February 2024 serves as a cautionary tale, illustrating the consequences of inadequate security measures and the importance of regular system updates. By incorporating expert advice and checklists for CSV, entities can ensure their systems remain compliant and secure.
By adhering to these practices, entities can improve their verification processes, guaranteeing alignment with Good Manufacturing Practices (GMP) and ISO standards. The significance of risk evaluation in cloud verification is further exemplified by case studies that demonstrate how entities prioritizing these assessments can effectively reduce risks and uphold strong regulatory frameworks.
Ensure Continuous Training and Support for Compliance Teams
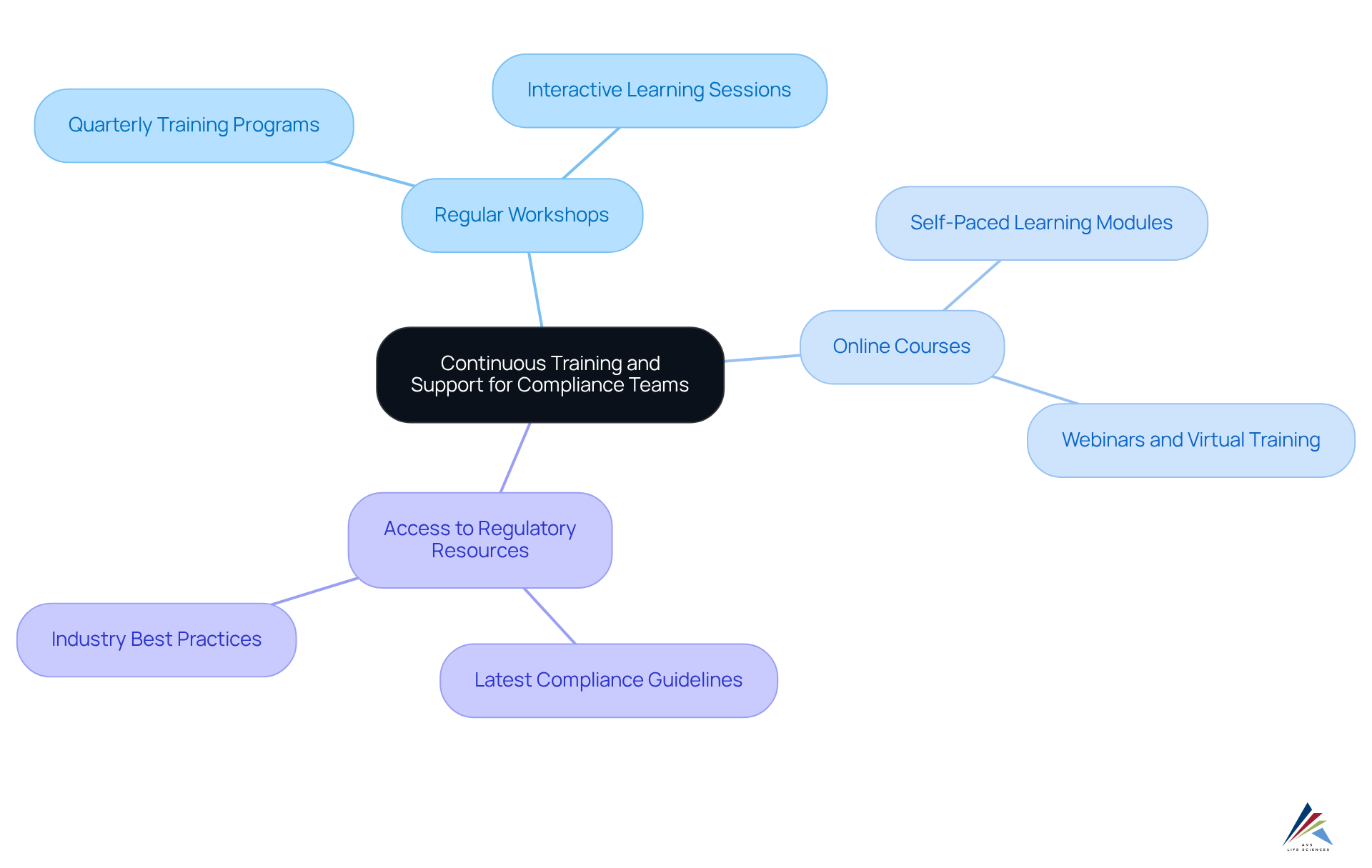
To empower regulatory teams in effectively managing cloud-based validation solutions, organizations must prioritize continuous training and support. This commitment can take the form of:
- Regular workshops
- Online courses
- Access to the latest regulatory resources
For example, a leading pharmaceutical firm developed a quarterly training program for its regulatory staff, which led to a significant reduction in audit findings and improved adherence outcomes. Furthermore, fostering a culture of open communication and collaboration within teams enhances knowledge sharing, thereby amplifying the success of cloud-based validation solutions. By investing in these training initiatives, organizations not only bolster their compliance capabilities but also cultivate a resilient workforce that is well-equipped to navigate the complexities of regulatory environments.
Conclusion
Cloud-based validation solutions represent a transformative approach within the life sciences sector, driving significant improvements in data accessibility, regulatory compliance, and operational efficiency. Embracing these innovative technologies enables organizations to enhance their validation processes, ensuring they meet stringent industry standards while remaining agile in a rapidly evolving landscape.
This article has explored the critical components of successful cloud-based validation, including:
- The identification of challenges during the transition
- The implementation of best practices
- The necessity for ongoing training and support for compliance teams
Conducting thorough risk assessments, establishing robust documentation practices, and leveraging automation are essential strategies for maintaining data integrity and compliance. Furthermore, fostering a culture of ongoing education equips teams to effectively navigate the complexities of regulatory requirements.
As the life sciences industry continues to evolve, adopting cloud-based validation solutions is not merely an option but a necessity for organizations aiming to thrive. By prioritizing these best practices and investing in workforce development, companies can ensure they are well-prepared to meet current and future validation challenges. The time to act is now—adopting these strategies will not only enhance compliance but also position organizations for sustainable success in an increasingly competitive environment.
Frequently Asked Questions
Why are cloud-based validation solutions important in life sciences?
Cloud-based validation solutions enhance data accessibility, collaboration, and compliance with standards such as GXP and FDA guidelines, which are critical for maintaining the integrity of verification processes.
How do cloud-based validation solutions improve data management?
They enable real-time data management, streamline updates, and offer scalability, allowing organizations to quickly adapt to evolving compliance requirements.
Can you provide an example of the benefits of cloud-based validation in the pharmaceutical industry?
A pharmaceutical firm that implemented cloud-based validation solutions achieved a 30% reduction in validation time, which expedited product launches while ensuring adherence to Good Manufacturing Practices (GMP).
How do cloud platforms enhance transparency in clinical trials?
Cloud platforms allow pharmaceutical companies to securely share anonymized trial information with oversight organizations, thereby enhancing transparency and compliance.
What role do Standard Operating Procedures (SOPs) play in cloud-based validation?
SOPs and robust documentation practices are essential in quality management solutions to ensure organizations meet and exceed industry standards.
How does AVS Life Sciences assist organizations in the life sciences sector?
AVS Life Sciences provides expert consulting, tailored methodologies, and services that enhance compliance and quality management in the life sciences domain.
