4 Key Practices for an Effective Regulatory Strategy

Overview
The article delineates four essential practices for formulating an effective regulatory strategy:
- Understanding regulatory frameworks
- Aligning strategies with product development goals
- Fostering cross-functional collaboration
- Implementing continuous monitoring and adaptation
Each practice underscores the imperative of integrating compliance throughout the product lifecycle, proactively identifying challenges, promoting teamwork across departments, and making ongoing adjustments to align with evolving regulations. This approach not only ensures organizational resilience but also enhances product quality, positioning the organization to effectively navigate the complexities of regulatory demands.
Introduction
Navigating the intricate landscape of regulatory compliance presents a formidable challenge for many organizations today. An effective regulatory strategy not only guarantees adherence to essential frameworks but also aligns seamlessly with product development goals, fostering innovation while upholding safety and quality standards. Yet, how can entities successfully integrate compliance into their operations and adapt to an ever-evolving regulatory environment? This article explores four key practices that empower organizations to construct a robust regulatory strategy, enhancing their capacity to meet compliance requirements while driving product success.
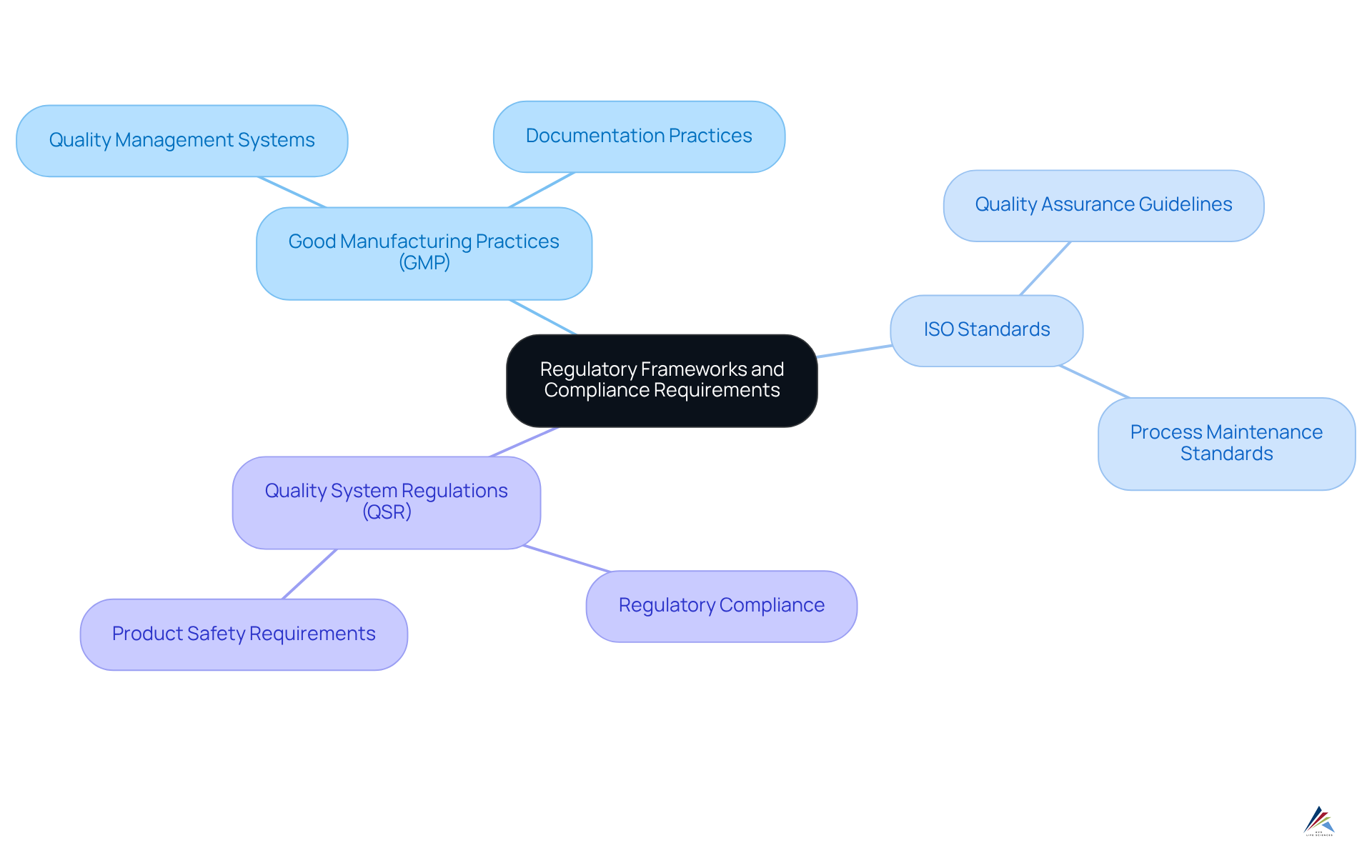
Understand Regulatory Frameworks and Compliance Requirements
Entities must first comprehend the regulatory frameworks that oversee their operations to create an effective regulatory strategy. This includes familiarizing themselves with:
Each of these frameworks outlines specific requirements that entities must adhere to in their regulatory strategy to ensure product safety, efficacy, and quality.
For instance, GMP emphasizes the importance of quality management systems and documentation practices, while ISO standards provide guidelines for maintaining quality assurance across various processes. Organizations should conduct thorough research and training to ensure that all team members are aware of these requirements, as can lead to significant legal and financial repercussions. Frequent assessments and evaluations of compliance practices can assist in recognizing deficiencies and opportunities for enhancement in the regulatory strategy, ensuring that the entity stays aligned with legal expectations.
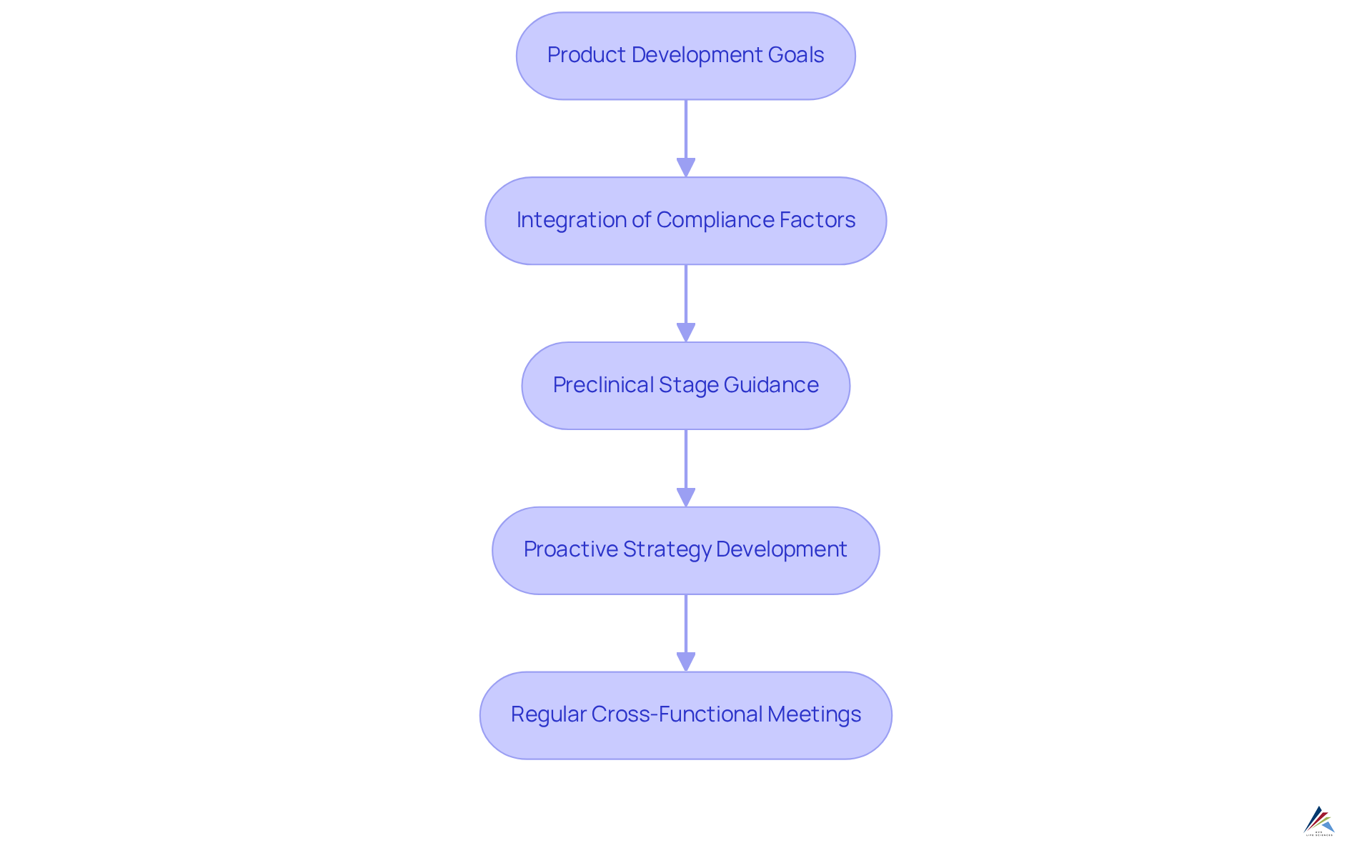
Align Regulatory Strategy with Product Development Goals
An effective regulatory strategy must closely align with the entity's product development objectives. This alignment necessitates the integration of compliance factors into the product lifecycle from the outset as part of a comprehensive regulatory strategy. By adopting this regulatory strategy, organizations can early identify potential compliance challenges and devise proactive strategies to address them.
For example, during the preclinical stage, compliance departments should provide guidance on the essential studies and documentation required for forthcoming submissions. This proactive strategy not only mitigates the risk of delays but also elevates the of the product. Establishing clear communication channels between compliance affairs and product development teams is crucial to ensure that all stakeholders are aligned regarding the regulatory strategy and compliance requirements. Regular cross-functional meetings can facilitate this alignment, enabling the identification of potential issues before they escalate.
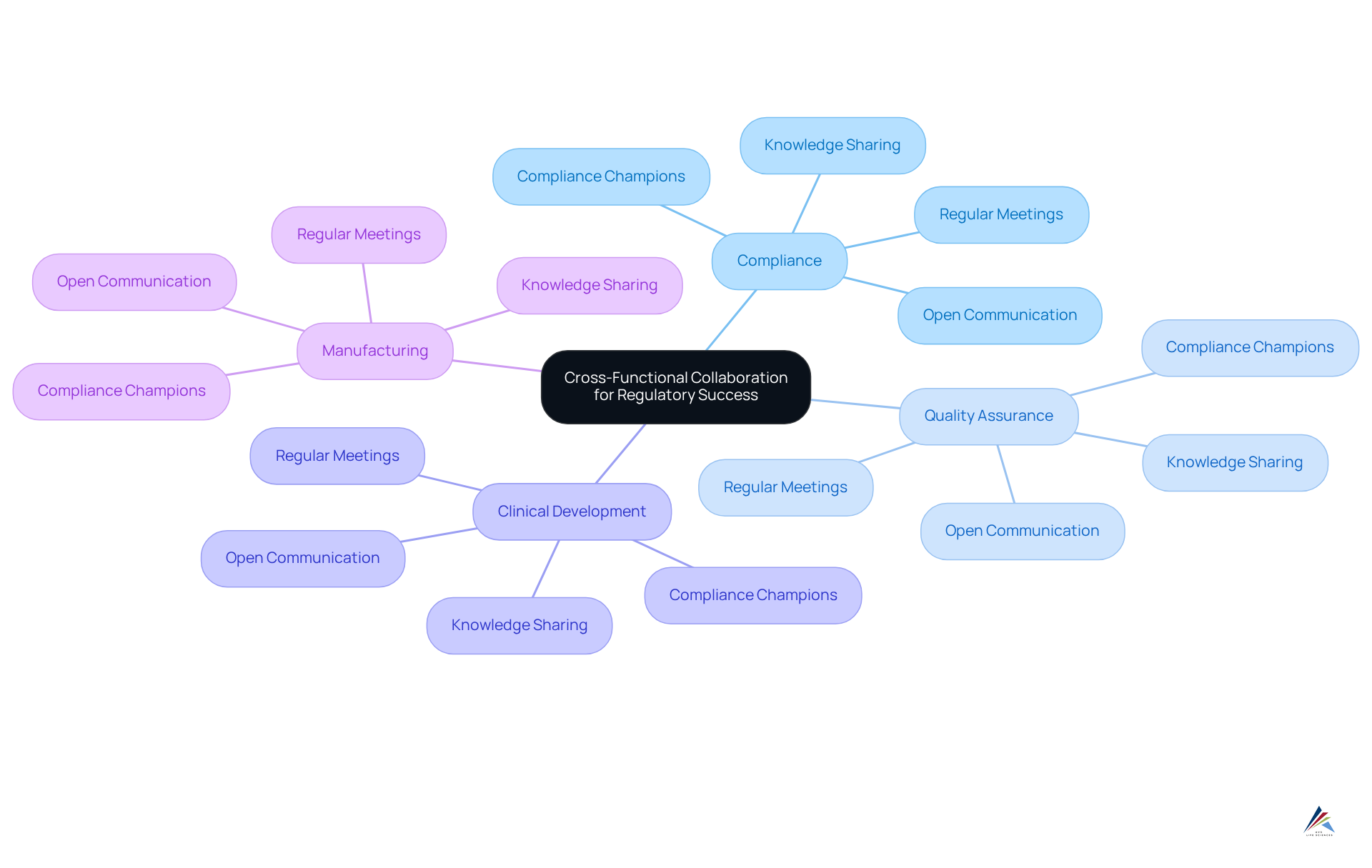
Foster Cross-Functional Collaboration for Regulatory Success
Encouraging cross-functional teamwork is vital for the success of our regulatory strategy. Organizations must foster collaboration among divisions such as:
- Compliance
- Quality assurance
- Clinical development
- Manufacturing
This partnership ensures that all teams are aligned and working towards common compliance objectives.
To facilitate this, companies should implement regular where members can share updates, discuss challenges, and brainstorm solutions. Furthermore, cultivating a culture of open communication and knowledge sharing is essential to dismantling silos and enhancing teamwork. For instance, appointing 'compliance champions' in every department can ensure that legal considerations are prioritized and integrated into daily operations. By leveraging the diverse expertise of various groups, organizations can strengthen their regulatory strategy and improve overall adherence outcomes.
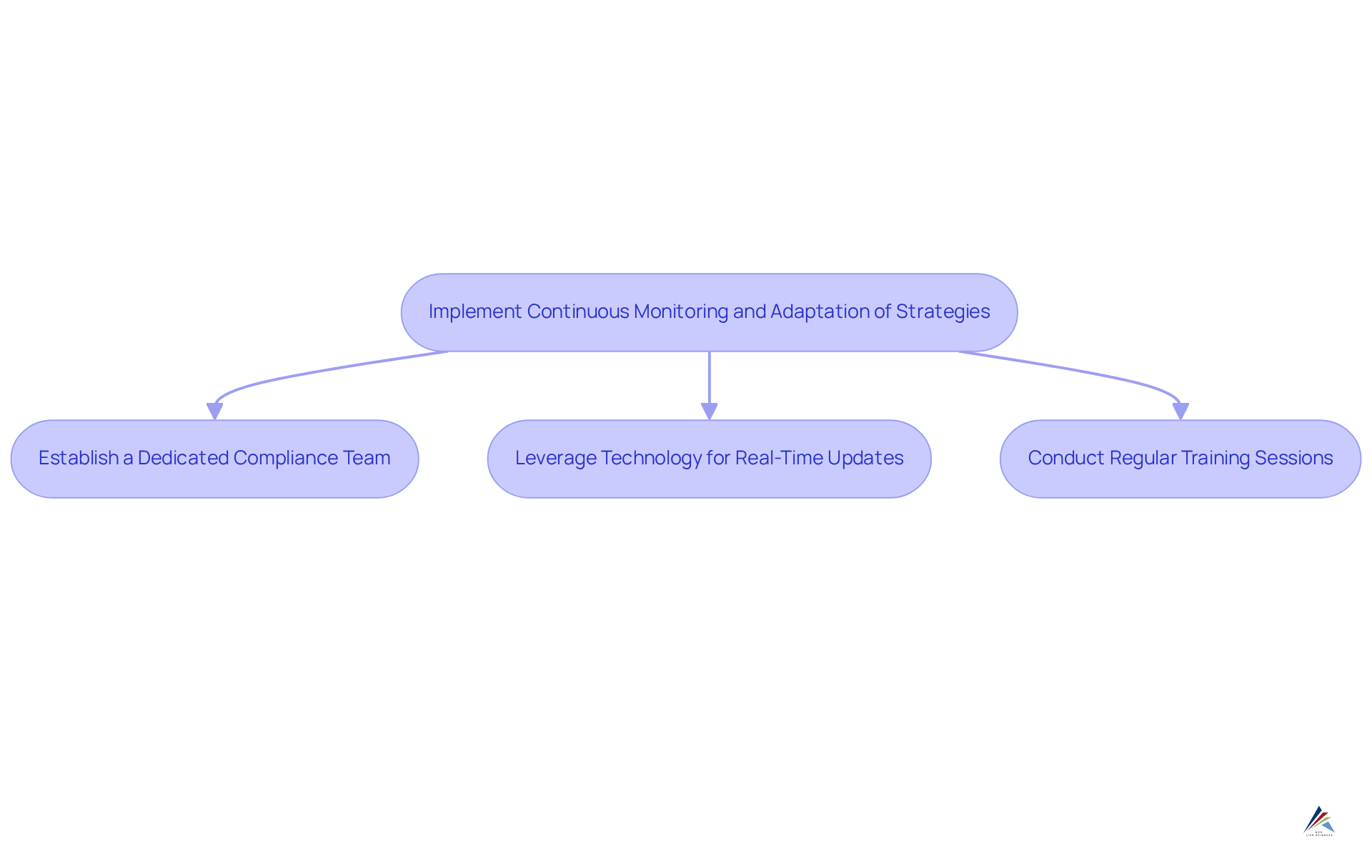
Implement Continuous Monitoring and Adaptation of Strategies
In today's dynamic compliance landscape, organizations face significant challenges that necessitate ongoing monitoring and adjustment of their oversight approaches. This requires a commitment to regularly reviewing and updating to align with evolving regulations, industry standards, and best practices.
To effectively navigate these complexities, organizations should:
- Establish a dedicated compliance team responsible for monitoring legal changes and assessing their impact on current strategies.
- Leverage technology, such as compliance management software, to streamline this process by providing real-time updates on legal developments.
- Conduct regular training sessions to ensure employees remain informed about changes in regulations and compliance requirements.
By fostering a culture of adaptability and vigilance, organizations can strengthen their regulatory strategy, ensuring it remains robust and effective against the backdrop of ever-evolving challenges.
Conclusion
Creating an effective regulatory strategy is essential for organizations aiming to navigate the complex landscape of compliance and product development. By understanding regulatory frameworks, aligning compliance with product goals, fostering cross-functional collaboration, and implementing continuous monitoring, entities can enhance their operational efficacy and mitigate risks associated with non-compliance.
The article highlights four key practices:
- Comprehending regulatory frameworks such as GMP, ISO standards, and QSR is crucial to ensure adherence to safety and quality requirements.
- Aligning regulatory strategies with product development from the outset allows organizations to proactively address compliance challenges.
- Fostering collaboration across departments encourages a unified approach to achieving compliance objectives.
- Continuous monitoring and adaptation of strategies ensure organizations remain responsive to evolving regulations and industry standards.
Ultimately, these practices underscore the importance of a proactive and integrated approach to regulatory strategy. Organizations that prioritize these elements not only safeguard their operations but also enhance their overall product quality and market readiness. Embracing these best practices will empower businesses to navigate the regulatory landscape more effectively, ensuring sustained compliance and success in an ever-changing environment.
Frequently Asked Questions
What are the key regulatory frameworks that entities must understand?
Entities must understand Good Manufacturing Practices (GMP), International Organization for Standardization (ISO) standards, and Quality System Regulations (QSR).
What is the importance of Good Manufacturing Practices (GMP)?
GMP emphasizes the importance of quality management systems and documentation practices to ensure product safety, efficacy, and quality.
How do ISO standards contribute to regulatory compliance?
ISO standards provide guidelines for maintaining quality assurance across various processes, helping organizations meet regulatory requirements.
What should organizations do to ensure compliance with regulatory frameworks?
Organizations should conduct thorough research and training to ensure that all team members are aware of regulatory requirements and practices.
What are the consequences of non-compliance with regulatory requirements?
Non-compliance can lead to significant legal and financial repercussions for the organization.
How can entities improve their regulatory strategy?
Frequent assessments and evaluations of compliance practices can help recognize deficiencies and opportunities for enhancement in the regulatory strategy.
