4 Essential Media Fill Practices for Compliance Officers

Overview
The article outlines four essential media fill practices that compliance officers must adopt to ensure adherence to regulatory standards and uphold product integrity in aseptic manufacturing processes. It underscores the significance of:
- Grasping media fill testing fundamentals
- Navigating regulatory requirements
- Implementing best practices
- Ensuring adequate personnel training
Each of these components is critical for validating the sterility of products and mitigating contamination risks. By understanding these practices, compliance officers can effectively address challenges and enhance operational integrity.
Introduction
Media fill testing serves as a cornerstone in pharmaceutical manufacturing, ensuring the production of sterile products within a contamination-free environment. For compliance officers, mastering the intricacies of media fill practices is not merely beneficial; it is essential for safeguarding product integrity and adhering to stringent regulatory requirements. As the landscape of regulations evolves, compliance officers face the challenge of effectively navigating the complexities of media fill protocols while upholding the highest standards of quality and safety. This article explores four essential media fill practices that empower compliance officers to enhance their processes and meet industry expectations.
Understand Media Fill Testing Fundamentals
Media fill testing is a crucial element in validating aseptic processes, as it is meticulously designed to demonstrate that the manufacturing environment and procedures can produce sterile products. This process involves simulating the media fill of a product with a growth substance, which is subsequently incubated to evaluate for microbial contamination. The key elements of this process are as follows:
- Purpose: The primary objective is to validate the sterility of the manufacturing process, ensuring that no contaminants are introduced during production.
- Methodology: This typically involves a media fill procedure, where vials or containers are filled with a sterile growth medium under aseptic conditions, followed by incubation and careful observation for any signs of microbial growth.
- Frequency: Regular media preparation tests are essential, particularly when changes occur in the manufacturing process or equipment.
Grasping these fundamentals is vital for compliance officers, empowering them to ensure that their organizations adhere to industry standards and uphold product integrity.
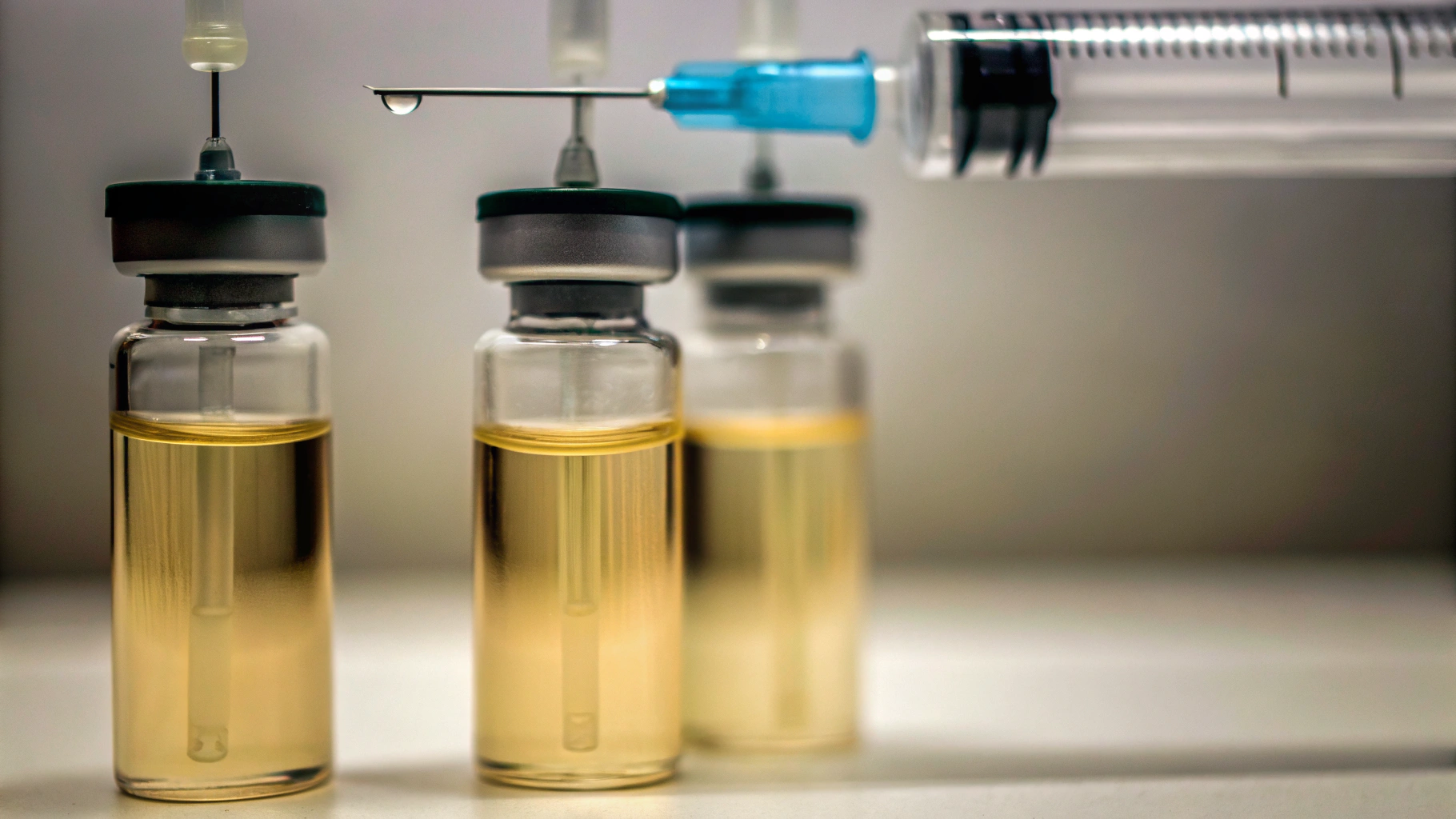
Navigate Regulatory Requirements for Media Fill Testing
Compliance officers must possess a comprehensive understanding of the regulatory environment governing media procedures, as adherence to established guidelines is crucial for maintaining product integrity and safety. Key regulations include:
-
FDA Guidance: The FDA outlines stringent requirements for media fill testing in its guidance documents, underscoring the necessity for robust validation of aseptic processes. Non-compliance in media preparation assessment can lead to significant regulatory repercussions, with data indicating that nearly 30% of establishments face issues related to inadequate validation methods. AVS Life Sciences offers services such as Environmental Monitoring Program Development and Quality Event Management to ensure compliance with these FDA requirements, facilitating a seamless integration of quality assurance throughout the drug development lifecycle.
-
European Medicines Agency (EMA): The EMA reflects the FDA's position by offering comprehensive guidelines for media fill testing in the EU. Recent updates from the EMA stress the importance of aligning with the new Variations Guidelines, which streamline the lifecycle management of medicines and enhance the efficiency of regulatory processes. AVS Life Sciences aids regulatory officers in adapting to these changes through comprehensive regulatory affairs services, including GXP Training and Inspection Readiness Support.
-
ISO Standards: Adhering to ISO standards, particularly ISO 13408, is imperative, as these standards provide a thorough framework for validating aseptic processes. Compliance with these standards not only ensures regulatory adherence but also fosters a culture of quality and safety within organizations. AVS Life Sciences delivers quality management systems development and implementation, ensuring that organizations meet these essential ISO standards.
By thoroughly understanding these regulatory requirements and leveraging the services of AVS Life Sciences, compliance officers can adeptly navigate the complexities of media fill protocols. This approach significantly mitigates the risk of regulatory non-compliance while ensuring the highest standards of product quality.

Implement Best Practices for Effective Media Fill Testing
To ensure effective media fill testing, compliance officers must adopt the following best practices:
-
Select Appropriate Growth Media: It is crucial to choose a growth medium that aligns with the product being tested, ensuring it can effectively support the growth of potential contaminants. The choice of growth substrates is essential, as it directly influences contamination levels and the overall dependability of the testing procedure.
-
Simulate Realistic Conditions: Media tests should be performed under circumstances that closely imitate actual production environments. This includes utilizing the same equipment and personnel to ensure that the tests reflect true operational scenarios. The FDA has emphasized the necessity of documenting smoke studies under dynamic conditions to demonstrate unidirectional flow and control over the aseptic process. A recent FDA Warning Letter highlighted shortcomings in processes due to unsanitary conduct observed during the procedure, underscoring the significance of adhering to Good Manufacturing Practices (GMP).
-
Maintain Comprehensive Documentation: It is vital to meticulously document every aspect of the media fill, including methodologies, results, and any deviations from standard operating procedures (SOPs). Recorded proof is a core tenet at the FDA, and detailed records are crucial for adherence and successful audits. Incorporating technical writing principles can enhance the clarity and professionalism of these documents. Upcoming training on documented evidence scheduled for 15.10.2025 will further support compliance officers in this area, reinforcing the critical nature of data integrity and CAPA in regulatory compliance.
-
Analyze Results Thoroughly: After incubation, a detailed analysis of the results must be conducted to identify any signs of contamination. This step is crucial for implementing corrective actions and ensuring that any issues are addressed promptly. Recent FDA alerts have emphasized the significance of assessing media outcomes, especially considering previous failures caused by unsanitary practices. The FDA has stated, "Too little smoke was produced to demonstrate the unidirectional flow," emphasizing the necessity for strict evaluation protocols and compliance with GXP standards.
By following these best practices, compliance officers can significantly enhance the efficiency of their media fill processes, ensuring conformity with industry standards and regulatory obligations.

Ensure Personnel Training and Qualification for Media Fill Testing
Guaranteeing that staff engaged in media preparation are adequately trained and qualified is crucial for preserving the integrity of the aseptic process. Key considerations include:
- Training Programs: Develop comprehensive training programs that encompass the fundamentals of aseptic techniques, media fill testing procedures, and relevant regulatory requirements. This foundational knowledge is crucial for effective execution.
- Regular Refresher Courses: Implement regular refresher courses to keep personnel informed about the latest industry practices and regulatory updates. Ongoing education assists in reducing the risk of outdated practices impacting regulations.
- Qualification Evaluations: Carry out comprehensive assessments to gauge personnel proficiency, ensuring they have the essential abilities to perform media fill accurately. These assessments are essential for upholding high standards of quality and adherence.
- Mentorship Opportunities: Pair less experienced staff with seasoned professionals to facilitate knowledge transfer and enhance practical skills. This mentorship approach not only builds confidence but also reinforces best practices in aseptic processing.
In light of the approximately 37% chance of contamination during sterile-to-sterile drug transfers, prioritizing personnel training and qualification is critical. Compliance officers can greatly decrease the risk of mistakes during media testing, ultimately improving overall adherence and protecting product integrity. As noted by industry experts, all personnel should have extensive training in aseptic practice before completing media fill qualification through aseptic process simulation. By integrating these practices, organizations can ensure a robust framework for maintaining aseptic integrity.

Conclusion
Understanding and implementing essential media fill practices is crucial for compliance officers responsible for ensuring the integrity of aseptic processes in pharmaceutical manufacturing. These practices not only validate product sterility but also assist organizations in navigating the intricate regulatory landscape, thereby safeguarding product quality and patient safety.
This article outlines the key components of media fill testing, emphasizing:
- The necessity of adhering to FDA and EMA guidelines.
- The importance of selecting appropriate growth media.
- The critical role of thorough training for personnel involved in the process.
By embracing best practices such as simulating realistic conditions and maintaining comprehensive documentation, compliance officers can significantly enhance the effectiveness of their media fill protocols, consequently reducing the risk of contamination and regulatory non-compliance.
Ultimately, the commitment to rigorous media fill testing and adherence to industry standards transcends mere compliance; it reflects a broader dedication to quality and safety in pharmaceutical manufacturing. As the landscape of regulatory requirements continues to evolve, it is imperative for compliance officers to remain informed and proactive, ensuring that their organizations not only meet but exceed the expectations set forth by governing bodies. Emphasizing training, documentation, and best practices will not only fortify compliance efforts but also contribute to the overarching goal of delivering safe and effective products to the market.
Frequently Asked Questions
What is media fill testing?
Media fill testing is a process used to validate aseptic processes by simulating the filling of a product with a growth substance to demonstrate that the manufacturing environment and procedures can produce sterile products.
What is the primary purpose of media fill testing?
The primary purpose of media fill testing is to validate the sterility of the manufacturing process, ensuring that no contaminants are introduced during production.
How is media fill testing conducted?
Media fill testing involves filling vials or containers with a sterile growth medium under aseptic conditions, followed by incubation and careful observation for any signs of microbial growth.
How often should media fill testing be conducted?
Regular media fill testing is essential, particularly when there are changes in the manufacturing process or equipment.
Why is understanding media fill testing fundamentals important for compliance officers?
Understanding media fill testing fundamentals is vital for compliance officers as it empowers them to ensure that their organizations adhere to industry standards and maintain product integrity.
