4 Best Practices for Effective Pharma Compliance Management

Overview
The article presents four best practices essential for effective pharma compliance management:
- Understanding regulatory frameworks
- Implementing systematic compliance strategies
- Prioritizing continuous training
- Leveraging technology
These practices address the significant compliance challenges faced by the pharmaceutical industry. By adhering to stringent regulations, organizations can not only improve operational efficiency but also enhance overall product quality and safety. Engaging with these strategies will empower compliance officers to navigate the complexities of the regulatory landscape confidently.
Introduction
Navigating the intricate landscape of pharmaceutical compliance presents a formidable challenge for organizations committed to upholding safety and quality standards. As regulatory frameworks continue to evolve, grasping the latest compliance requirements becomes essential for sustaining operational excellence and protecting patient health.
How can pharmaceutical companies not only adhere to these stringent regulations but also harness them as a catalyst for innovation and growth? This article delves into four best practices for effective pharma compliance management, providing insights into strategies that can transform compliance from a burden into a competitive advantage.
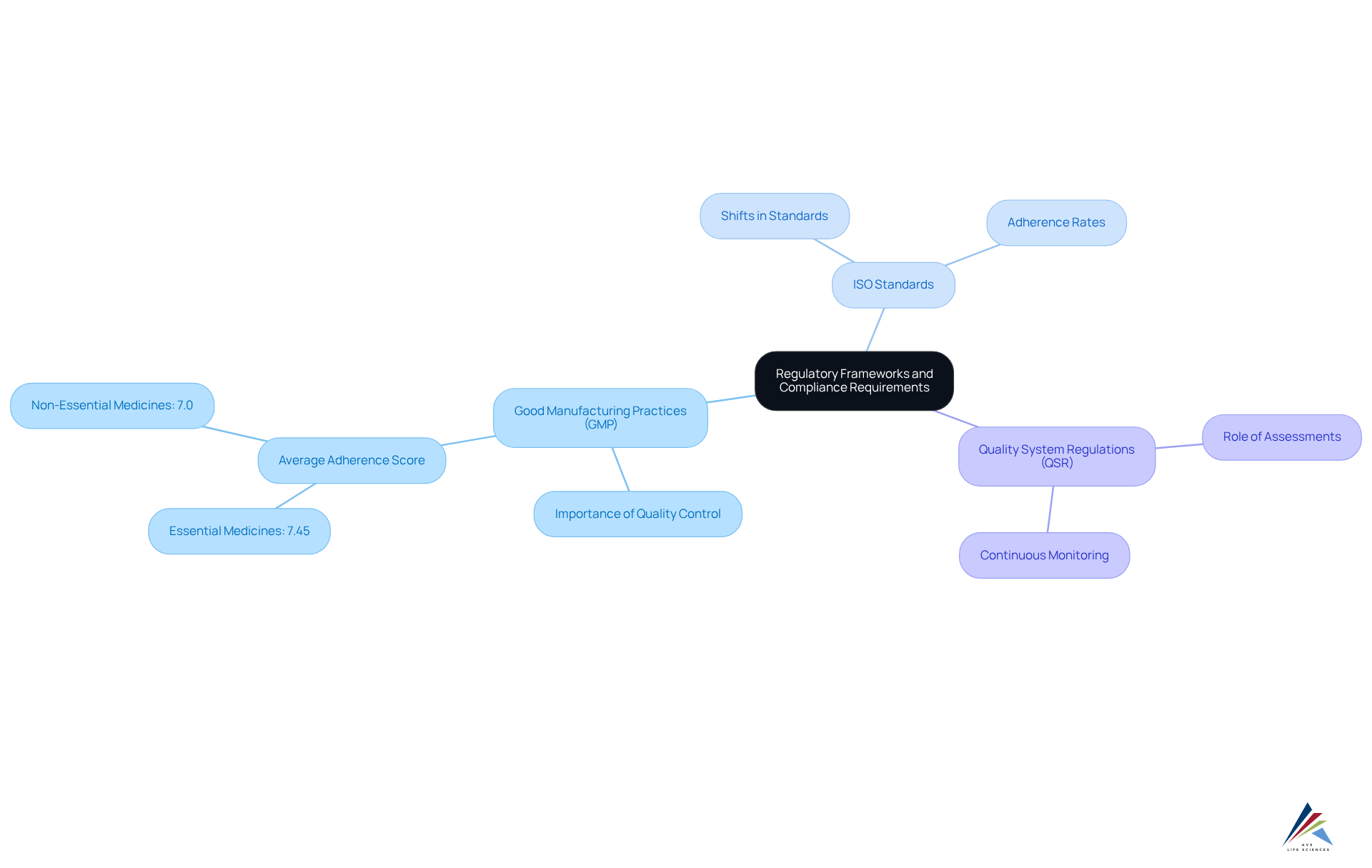
Understand Regulatory Frameworks and Compliance Requirements
To effectively manage adherence, organizations must thoroughly understand the regulatory frameworks governing their operations, including:
Each framework outlines specific requirements essential for ensuring product safety, efficacy, and quality. For instance, GMP emphasizes the necessity of maintaining consistent manufacturing processes and robust quality control measures.
In 2025, the pharmaceutical sector is experiencing notable shifts in ISO standards, with adherence rates illustrating an increasing dedication to quality—essential medicines producers attained an average GMP adherence score of 7.45 out of 10, whereas non-essential medicines scored 7.0. Companies should perform regular assessments of these regulations and remain informed about any updates to ensure continuous adherence.
Interacting with oversight organizations and sector groups can offer important perspectives on changing adherence standards, promoting a proactive stance towards quality control. Industry leaders stress that pharma compliance with these standards is not just a legal requirement but a fundamental aspect of operational excellence and patient safety.
AVS Life Sciences offers extensive quality management and adherence solutions tailored to meet these evolving standards, ensuring pharma compliance and enabling entities to not only conform but thrive in their operational practices.
Implement Systematic Compliance Strategies and Best Practices
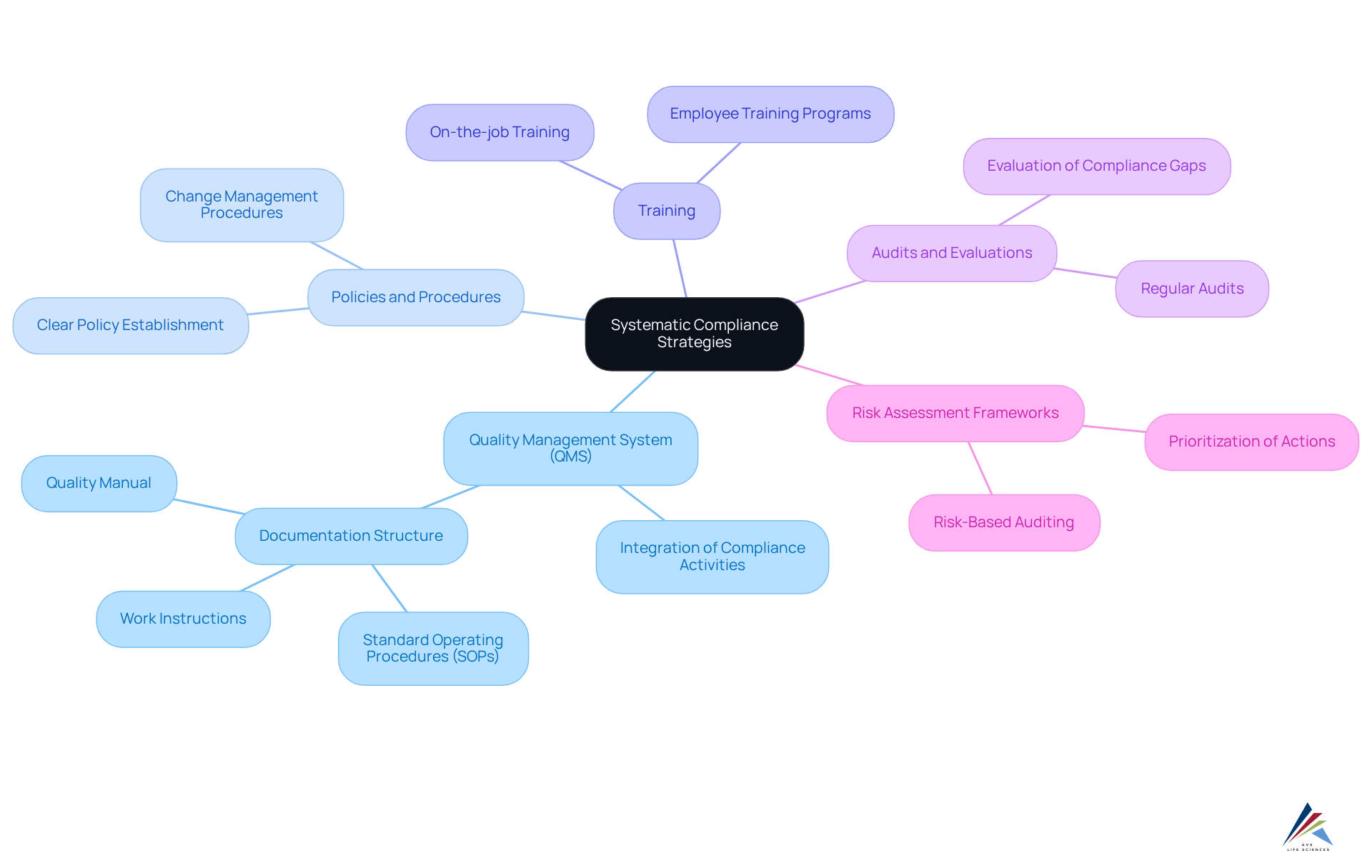
Implementing systematic adherence strategies necessitates a structured approach to managing regulations across the organization. A comprehensive Quality Management System (QMS) is indispensable for ensuring pharma compliance, integrating all compliance-related activities and aligning with regulatory requirements, including GXP and FDA regulations. Organizations must establish clear policies and procedures, guaranteeing that all employees receive thorough training on these protocols, including Standard Operating Procedures (SOPs).
Regular audits and evaluations are crucial for identifying regulatory gaps in pharma compliance and addressing them promptly. Moreover, employing risk assessment frameworks can enhance adherence efforts by prioritizing actions based on the potential impact and probability of non-conformity. For instance, a pharmaceutical company might adopt a risk-based approach, concentrating audits on high-risk areas such as manufacturing and distribution. This proactive strategy not only mitigates risks but also cultivates a culture of , which is essential for maintaining high standards of pharma compliance in the rapidly evolving pharmaceutical landscape.
As W. Edwards Deming aptly stated, 'Quality comes not from inspection, but from improvement of the production process,' underscoring the necessity for continuous enhancement in regulatory strategies. Furthermore, an effectively organized QMS typically encompasses a quality manual, policies, SOPs, work instructions, and records, which are vital for effective documentation and regulatory management.
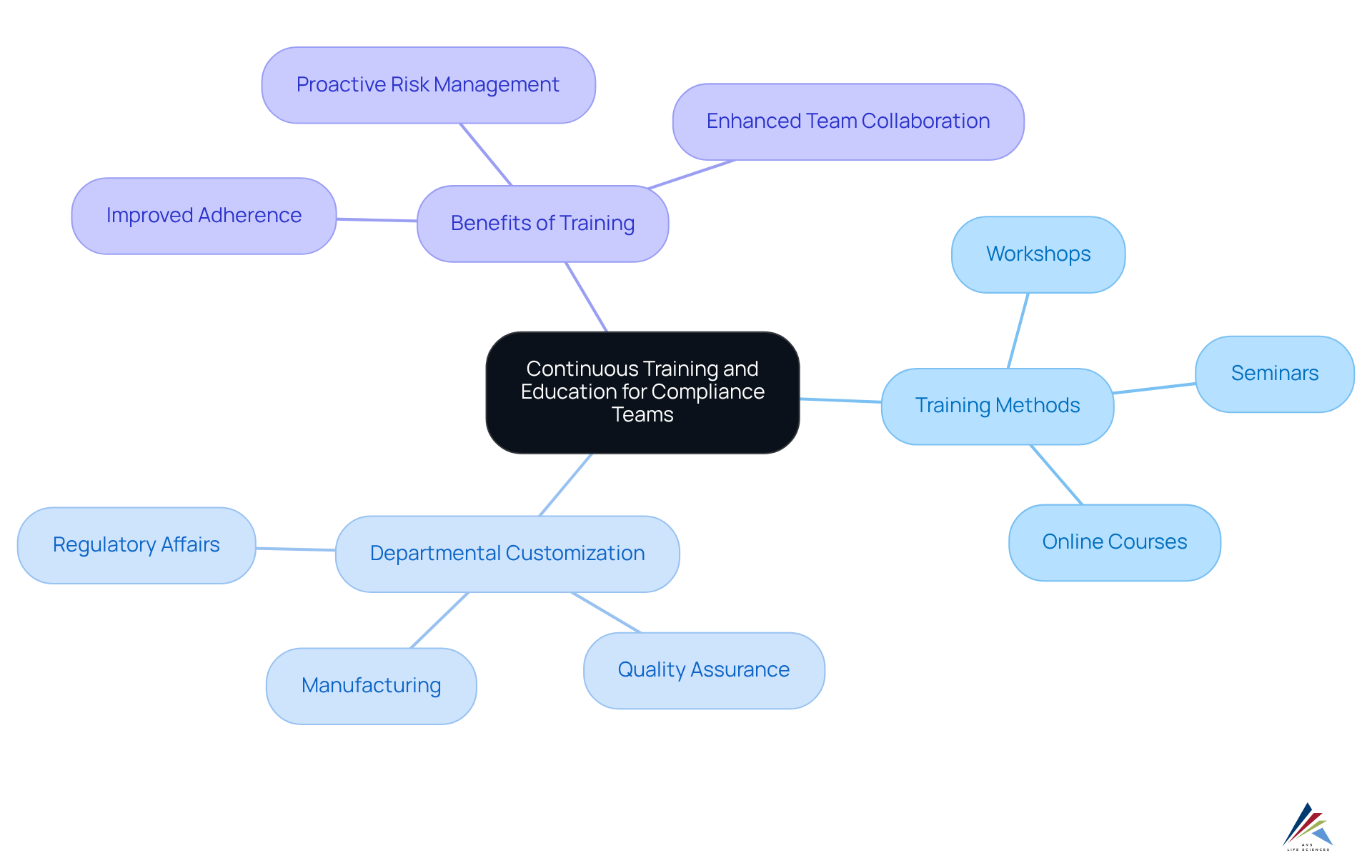
Prioritize Continuous Training and Education for Compliance Teams
To uphold rigorous standards of pharma compliance, organizations must prioritize for their regulatory teams. This includes:
- Regular workshops
- Seminars
- Online courses that cover the latest policy updates and best practices
Customizing training sessions to address specific regulatory challenges faced by departments such as manufacturing, quality assurance, and regulatory affairs can significantly enhance understanding and responsiveness. For instance, cross-training initiatives can cultivate a culture of adherence across the organization, enabling teams to share insights and strategies effectively.
By investing in employee development, organizations not only bolster their regulatory capabilities but also empower their teams to proactively identify and address risks. Ongoing education in pharma compliance has been shown to improve adherence effectiveness, ensuring that staff remain informed and prepared to navigate the evolving legal landscape.
Leverage Technology for Enhanced Compliance Management
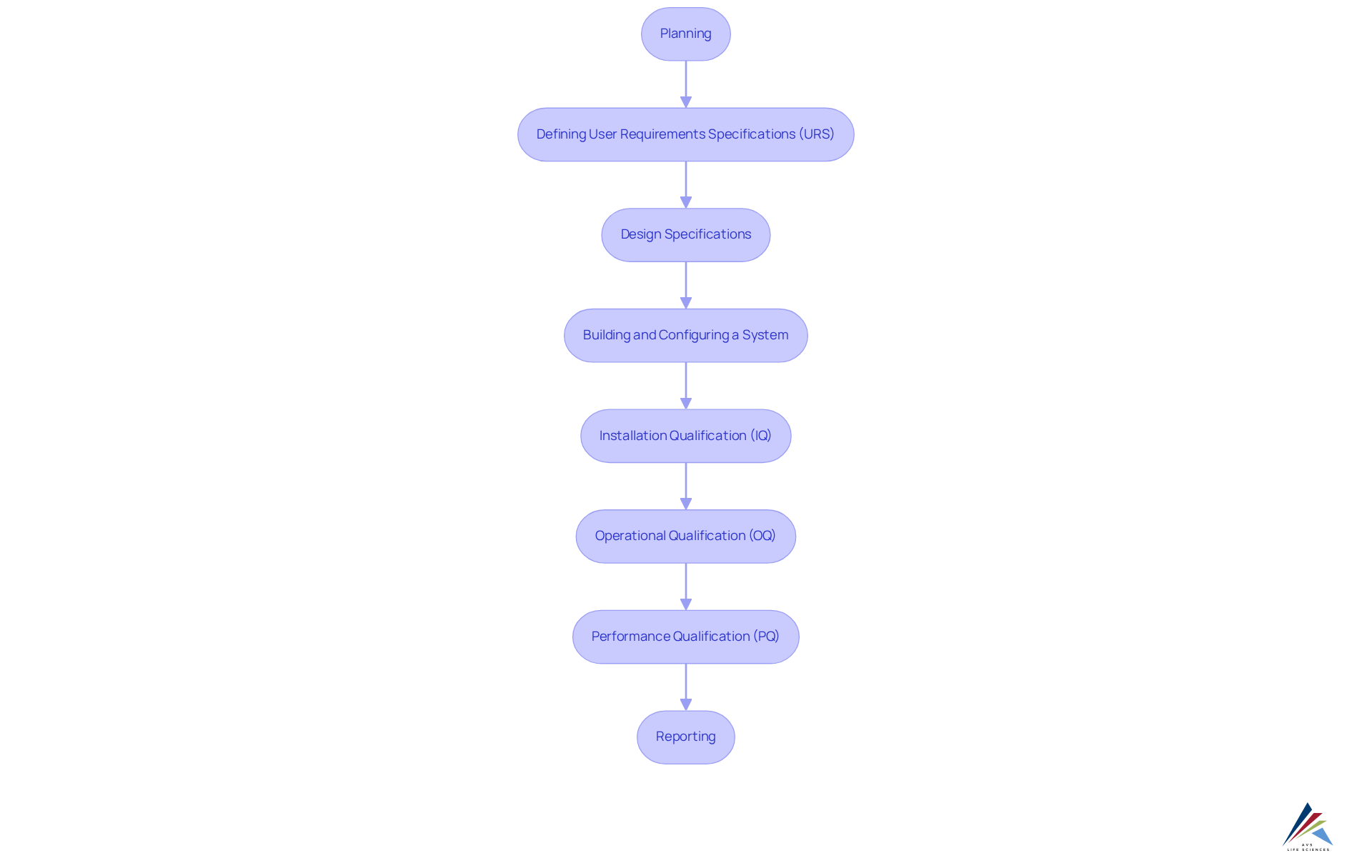
In the pharmaceutical sector, the utilization of technology is essential for enhancing compliance oversight. Compliance oversight software automates routine tasks such as document handling, audit tracking, and compliance reporting, ensuring that all compliance-related activities are accurately recorded and readily accessible for audits. A comprehensive approach to computer system validation (CSV) is vital, encompassing multiple stages, including:
- Planning: The management of the entity establishes the required budget and timeline for executing computer system validation.
- Defining User Requirements Specifications (URS): This encompasses the tasks that a system must be able to perform.
- Design Specifications: A team decides on the appearance and operation of functions to meet the requirements.
- Building and Configuring a System: Development of configured scripts for software design.
- Installation Qualification (IQ): Testing to ensure the appropriate installation method is selected.
- Operational Qualification (OQ): Testing to confirm the system operates as intended in the user environment.
- Performance Qualification (PQ): Simulating worst-case scenarios to ensure continued functionality under adverse conditions.
- Reporting: Documenting all actions executed during IQ, OQ, and PQ testing.
For instance, organizations can leverage predictive analytics to track adherence metrics and identify patterns that may indicate potential challenges, allowing them to proactively adjust their strategies in response to anticipated policy shifts. This technological integration not only enhances operational efficiency but also significantly mitigates the risk of with pharma compliance regulations, which can lead to severe penalties and lost marketing opportunities. Consequently, organizations can bolster their overall adherence to regulations, ensuring they meet stringent regulatory requirements while upholding high standards of quality and safety. The pharmaceutical regulatory software market is projected to grow from USD 1.5 billion in 2023 to approximately USD 3.2 billion by 2032, reflecting a compound annual growth rate (CAGR) of 8.2%. Tools such as Veeva Vault RIM Suite and MasterControl exemplify how regulatory management software can improve audit tracking and reporting. However, organizations must also remain vigilant regarding common pitfalls in implementing these solutions, such as integration challenges and the necessity for ongoing training, to avoid missteps in their compliance strategies.
Conclusion
In the realm of pharmaceutical compliance management, a robust understanding of regulatory frameworks, systematic strategies, ongoing training, and technological integration emerges as essential practices for success. By prioritizing these elements, organizations can not only meet compliance requirements but also enhance their operational excellence and commitment to patient safety.
Familiarizing oneself with key regulations such as:
- GMP
- ISO standards
- QSR
is paramount. Systematic compliance strategies through comprehensive Quality Management Systems are advocated to ensure robust adherence. Regular training for compliance teams stands out as a crucial factor in maintaining high adherence standards, equipping staff to navigate the evolving legal landscape effectively. Furthermore, leveraging technology for compliance management streamlines processes and mitigates risks associated with non-compliance.
The significance of these best practices cannot be overstated. As the pharmaceutical industry continues to evolve, organizations must remain vigilant in their adherence efforts. By embracing these strategies, companies can fulfill regulatory obligations while fostering a culture of continuous improvement and innovation that ultimately benefits both their operations and the patients they serve.
Frequently Asked Questions
What are the key regulatory frameworks that organizations need to understand for compliance?
Organizations must understand Good Manufacturing Practices (GMP), International Organization for Standardization (ISO) standards, and Quality System Regulations (QSR) to effectively manage adherence.
What is the significance of Good Manufacturing Practices (GMP)?
GMP emphasizes the necessity of maintaining consistent manufacturing processes and robust quality control measures, which are essential for ensuring product safety, efficacy, and quality.
How are ISO standards changing in the pharmaceutical sector by 2025?
By 2025, the pharmaceutical sector is experiencing notable shifts in ISO standards, with increasing adherence rates indicating a growing dedication to quality. Essential medicines producers achieved an average GMP adherence score of 7.45 out of 10, while non-essential medicines scored 7.0.
Why is it important for companies to perform regular assessments of regulations?
Regular assessments of regulations are important for companies to remain informed about any updates, ensuring continuous adherence to compliance standards.
How can engaging with oversight organizations and sector groups benefit companies?
Interacting with oversight organizations and sector groups can provide important perspectives on changing adherence standards, promoting a proactive approach to quality control.
What do industry leaders say about compliance with these standards?
Industry leaders emphasize that compliance with these standards is not just a legal requirement but also a fundamental aspect of operational excellence and patient safety.
What solutions does AVS Life Sciences offer to help with compliance?
AVS Life Sciences provides extensive quality management and adherence solutions tailored to meet evolving standards, ensuring pharmaceutical compliance and helping entities thrive in their operational practices.
