3 Key Strategies for Effective Regulatory Labeling Compliance

Overview
Achieving effective regulatory labeling compliance requires a strategic approach centered around three essential strategies:
- Understanding regulatory requirements
- Implementing best practices
- Monitoring regulatory changes
Organizations face significant compliance challenges in the ever-evolving landscape of life sciences. To navigate these complexities, it is imperative for them to:
- Stay informed about labeling requirements
- Standardize their processes
- Leverage technology to adapt to regulatory shifts
Such proactive measures not only enhance safety but also ensure compliance within the sector. By embracing these strategies, organizations can position themselves as leaders in regulatory compliance, ultimately fostering a culture of safety and reliability.
Introduction
Navigating the intricate landscape of regulatory labeling compliance poses a formidable challenge for organizations in the life sciences sector. As standards evolve under the guidance of authorities such as the FDA and EMA, grasping the nuances of labeling requirements becomes paramount for ensuring product safety and market viability. This article delves into three key strategies designed to enhance compliance and empower businesses to adapt swiftly to regulatory changes.
How can organizations effectively implement these practices to safeguard against potential pitfalls and elevate their labeling processes?
Understand Regulatory Labeling Requirements
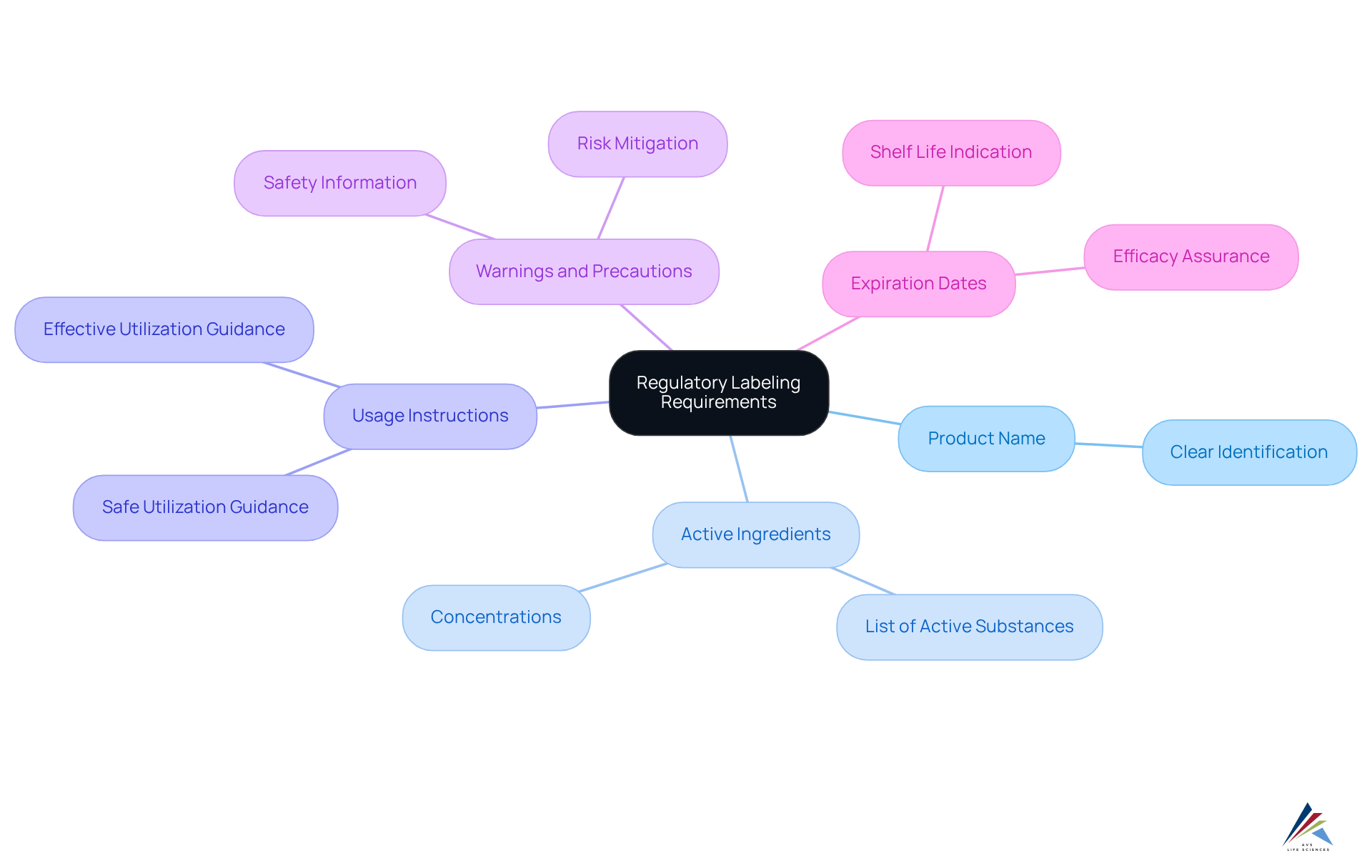
To achieve compliance with , organizations must thoroughly understand the specific requirements established by regulatory authorities such as the FDA and EMA. Key components of product labels include:
- Product Name: A clear identification of the product is essential for consumer recognition.
- Active Ingredients: All active substances must be listed along with their concentrations to ensure transparency.
- Usage Instructions: Detailed guidance on safe and effective utilization is crucial for consumer safety.
- Warnings and Precautions: Important safety information must be clearly communicated to mitigate risks associated with usage.
- Expiration Dates: A clear indication of the product's shelf life is necessary to ensure efficacy and safety.
Staying updated on regulatory labeling compliance changes is essential, as these can greatly influence marking requirements. Regular training sessions and workshops can enhance staff knowledge of the latest developments in regulatory standards. For instance, the EMA's revised regulatory labeling standards for 2025 highlight the necessity for clarity and precision in regulatory labeling to prevent typical mistakes that result in problems. Entities that emphasize grasping these needs not only boost their adherence but also enhance their overall safety and market appeal.
AVS Life Sciences provides extensive quality management and regulatory adherence solutions that can help organizations navigate these complex requirements. By utilizing our knowledge, businesses can guarantee their marking practices comply with the latest standards, thus improving safety and market appeal.
Implement Best Practices for Labeling Compliance
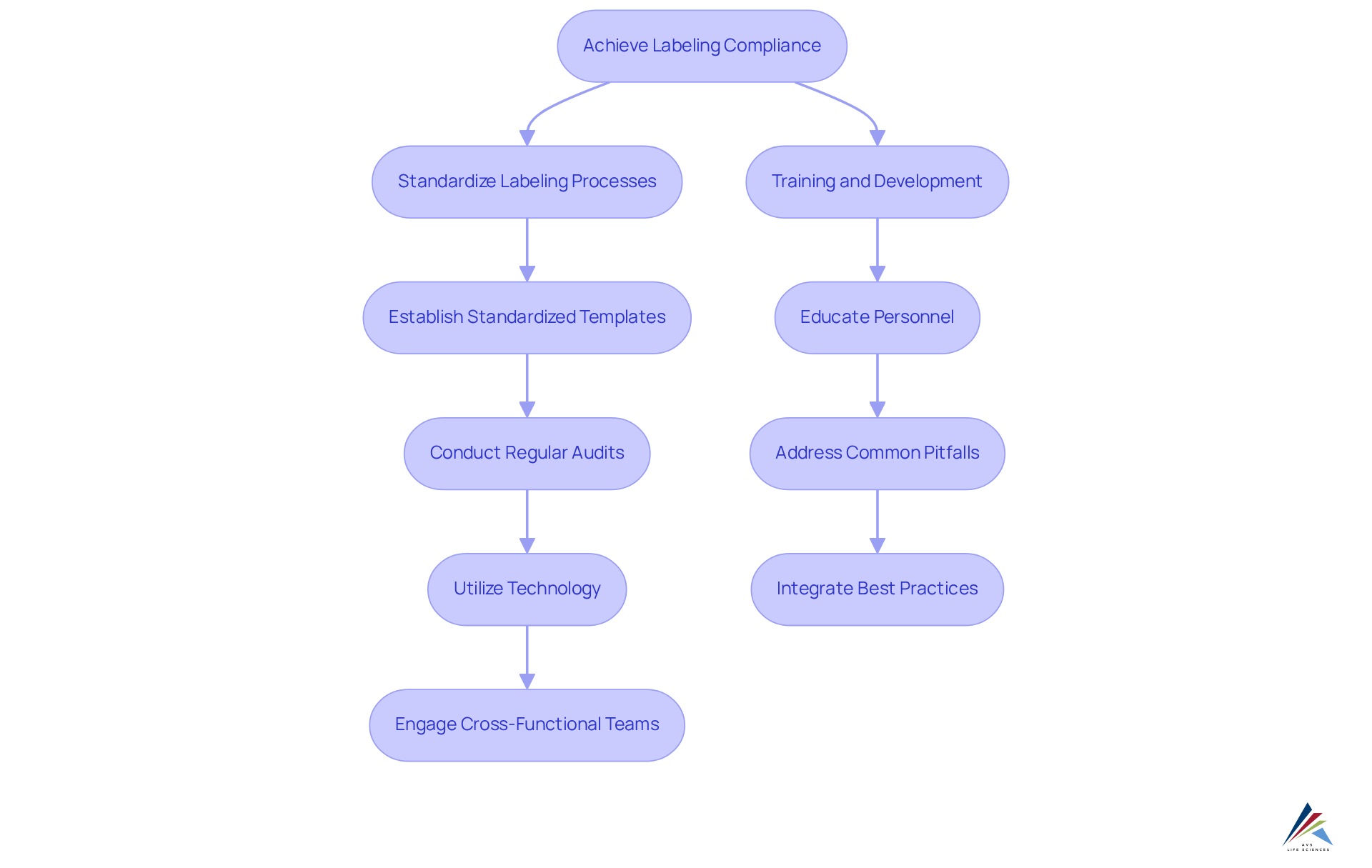
To achieve labeling compliance, organizations must adopt the following best practices:
- Standardize Labeling Processes: Establish standardized templates and procedures for label creation to ensure consistency and compliance across all products, particularly in biopharmaceuticals and nutraceuticals. This method not only improves efficiency but also significantly reduces the risk of mistakes. Industry specialists emphasize that applying standardized measures can greatly decrease errors in identification.
Conduct regular audits to evaluate regulatory labeling practices and identify any discrepancies or non-compliance issues before they escalate. Regular audits uphold compliance with industry standards and regulatory labeling while fostering a proactive culture of adherence, which is crucial for organizations in the biopharmaceutical and nutraceutical fields.
Utilize technology by leveraging advanced tagging software that incorporates and automates updates, ensuring tags remain aligned with the latest standards. The application of technology simplifies the tagging process, enabling faster modifications in response to compliance changes, a crucial element of AVS Life Sciences' extensive compliance solutions.
Engage cross-functional teams by collaborating with marketing, legal, and regulatory affairs teams to ensure that all aspects of product information, including regulatory labeling, are considered and compliant. This cross-departmental involvement encourages a comprehensive approach to identification, addressing potential regulatory labeling challenges from various viewpoints, which is essential for upholding quality standards in the life sciences sector. - Training and Development: Regularly educate personnel on tagging requirements and best practices to cultivate a culture of adherence within the organization. Ongoing education enables employees to remain knowledgeable about changing regulations and improves overall accuracy, in line with AVS Life Sciences' dedication to professional consulting in quality adherence.
Organizations should be aware of common pitfalls in regulatory labeling, such as neglecting to update labels in response to compliance changes or failing to involve all relevant departments in the process. For instance, a failure to refresh ingredient lists on nutraceutical products can lead to substantial regulatory problems. Addressing these challenges proactively can assist in upholding standards and minimizing the risk of mistakes, ultimately enhancing patient safety and ensuring compliance with regulatory labeling.
Integrating these optimal methods can significantly improve marking adherence and reduce the chances of mistakes, ultimately contributing to better patient safety and compliance with regulatory labeling requirements. This approach strengthens AVS Life Sciences' position as a leading provider of quality management and compliance solutions.
Monitor and Adapt to Regulatory Changes
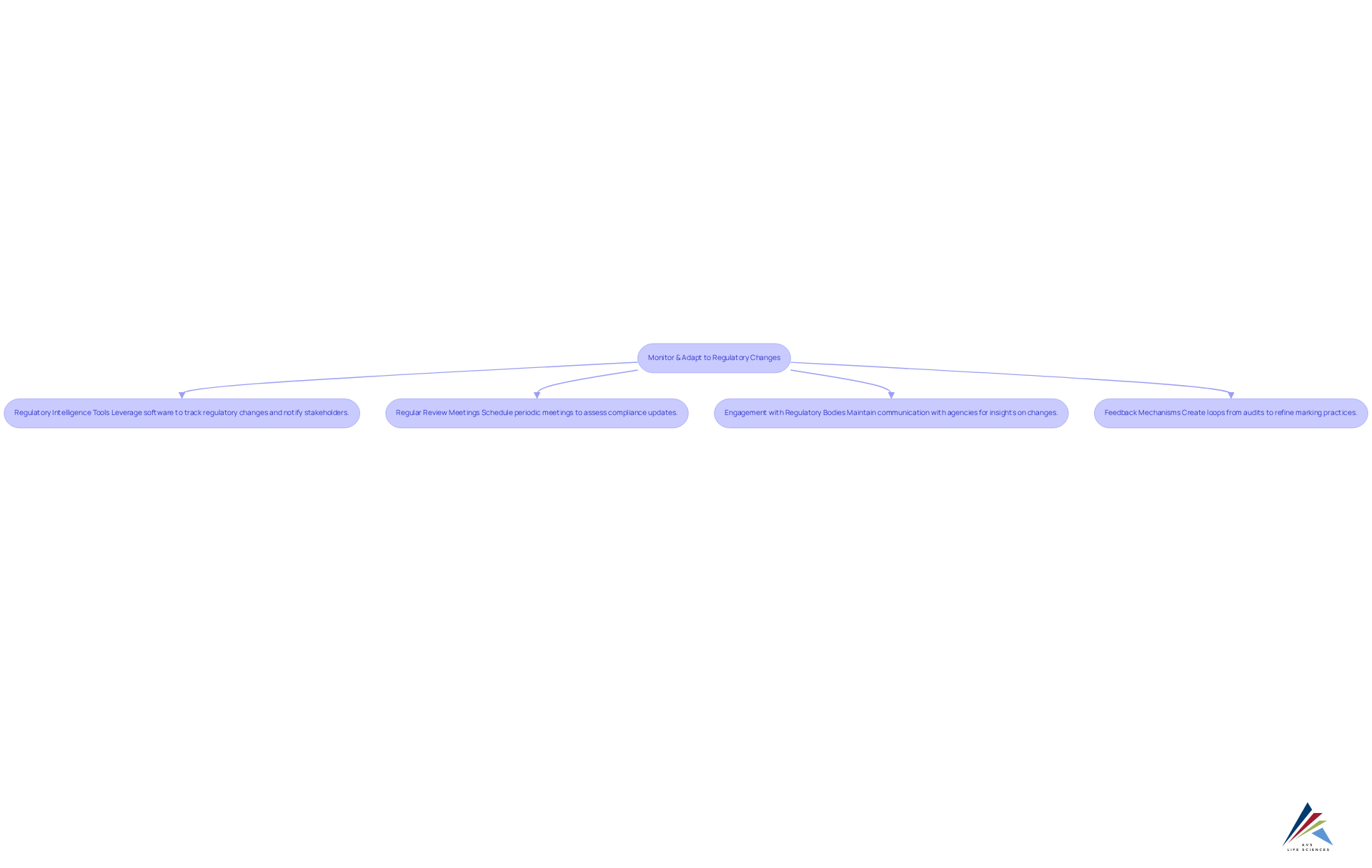
To ensure adherence to marking standards, organizations must implement a . This can be effectively accomplished through several key strategies:
- Regulatory Intelligence Tools: Leverage advanced software solutions designed to track regulatory changes and notify relevant stakeholders of updates that could impact labeling practices. These tools provide real-time insights, enabling organizations to stay ahead of regulatory requirements.
- Regular Review Meetings: Establish a schedule for periodic gatherings focused on assessing compliance updates. These sessions should evaluate the implications of changes on current marking practices, fostering a proactive approach to compliance.
- Engagement with Regulatory Bodies: Maintain proactive communication with regulatory agencies to stay informed about forthcoming changes. Engaging in discussions with these bodies can yield valuable insights that may influence future regulations and help organizations prepare accordingly.
- Feedback Mechanisms: Create feedback loops from audits and inspections to continuously refine marking practices. Applying knowledge gained from these assessments can enhance adherence and ensure that descriptions effectively convey crucial product details.
By actively monitoring and adapting to regulatory changes, organizations can maintain compliance and enhance the effectiveness of their regulatory labeling strategies.
Conclusion
Achieving effective regulatory labeling compliance transcends mere legal obligations; it stands as a cornerstone for ensuring consumer safety and preserving market integrity. Organizations must prioritize a thorough understanding of labeling requirements, adopt best practices, and vigilantly monitor regulatory changes to strengthen their compliance initiatives. By doing so, they can substantially mitigate the risk of errors and enhance their reputation within the industry.
The article delineates three pivotal strategies:
- Comprehending regulatory requirements
- Implementing best practices
- Monitoring changes
By emphasizing the necessity for clarity in product labeling, organizations can guarantee that critical information is communicated effectively, thus fostering consumer trust. Standardizing processes and involving cross-functional teams are essential steps that cultivate a compliance-oriented culture. Moreover, harnessing technology and regulatory intelligence tools equips organizations with the insights needed to remain ahead of evolving regulations.
Ultimately, organizations that proactively tackle regulatory labeling compliance not only protect their products but also contribute to the broader safety and well-being of consumers. By adopting these strategies and committing to continuous education and adaptation, businesses can adeptly navigate the complexities of regulatory requirements, ensuring they remain compliant and competitive in a constantly changing landscape.
Frequently Asked Questions
What are the key components of product labels required for regulatory compliance?
Key components of product labels include the product name, active ingredients with their concentrations, usage instructions, warnings and precautions, and expiration dates.
Why is it important to understand regulatory labeling requirements?
Understanding regulatory labeling requirements is essential to ensure compliance with authorities like the FDA and EMA, which helps to enhance consumer safety and product efficacy.
How can organizations stay updated on changes in regulatory labeling compliance?
Organizations can stay updated by participating in regular training sessions and workshops that focus on the latest developments in regulatory standards.
What are the implications of the EMA's revised regulatory labeling standards for 2025?
The EMA's revised standards emphasize the necessity for clarity and precision in regulatory labeling to prevent common mistakes that can lead to compliance issues.
How can AVS Life Sciences assist organizations with regulatory labeling compliance?
AVS Life Sciences provides quality management and regulatory adherence solutions that help organizations navigate complex labeling requirements and ensure compliance with the latest standards.
