3 Key Steps for Digital Validation Implementation in Pharma

Overview
The article delineates three pivotal steps for implementing digital validation in the pharmaceutical industry:
- Defining objectives
- Selecting suitable instruments
- Developing a transition strategy
These steps are crucial for ensuring compliance and operational efficiency, as they offer a structured methodology for adopting electronic verification tools that align with regulatory standards and enhance product development processes. By addressing compliance challenges head-on, this framework not only streamlines operations but also fosters a culture of accountability and innovation within the industry.
Introduction
The pharmaceutical industry is experiencing a profound transformation as it embraces digital validation, a critical process for ensuring compliance and enhancing operational efficiency. By leveraging electronic verification tools, organizations can significantly improve data integrity and streamline their compliance efforts, ultimately resulting in accelerated product development cycles. However, as the sector navigates the complexities of regulatory requirements, one pivotal question arises: what essential steps must pharmaceutical companies undertake to effectively implement digital validation and navigate the challenges that lie ahead?
Understand Digital Validation in Pharmaceutical Compliance
Electronic verification is essential for ensuring that electronic systems and processes within the pharmaceutical sector function correctly and adhere to industry standards. This critical process involves the use of software tools and methodologies to validate computerized systems, thereby guaranteeing data integrity, accuracy, and reliability. Key components of digital validation include:
- Risk assessment
- Thorough documentation
- Strict adherence to regulatory guidelines such as 21 CFR Part 11 and EU GMP Annex 11
Mastering these elements is vital for organizations transitioning from traditional validation methods to more efficient digital solutions, ultimately enhancing compliance and operational efficiency.
The practical applications of electronic verification tools have led to significant improvements in assessment procedures. For example, a pharmaceutical firm that adopted an electronic verification system experienced an impressive 30% reduction in verification time. This efficiency not only accelerated product development cycles but also improved audit outcomes, demonstrating the on compliance within the pharmaceutical sector.
Furthermore, AI-assisted verification has the potential to preserve entire batches and significantly reduce deviation investigation time, underscoring the efficiency gains from these tools. As the sector evolves in 2025, the integration of electronic verification will be crucial in navigating the complexities of regulatory obligations and ensuring robust adherence. The current landscape also presents challenges, such as a shortage of human resources, identified as a primary verification hurdle by industry surveys. Additionally, organizations like the FDA and EMA expect comprehensive electronic audit trails as evidence of data integrity, highlighting the importance of compliance in electronic assessment procedures.
AVS Life Sciences offers comprehensive solutions that address these challenges, including strategies for managing data integrity deviations, conducting investigations, and implementing CAPA processes, ensuring that organizations can adeptly navigate the complexities of electronic verification.

Implement Effective Digital Validation Strategies
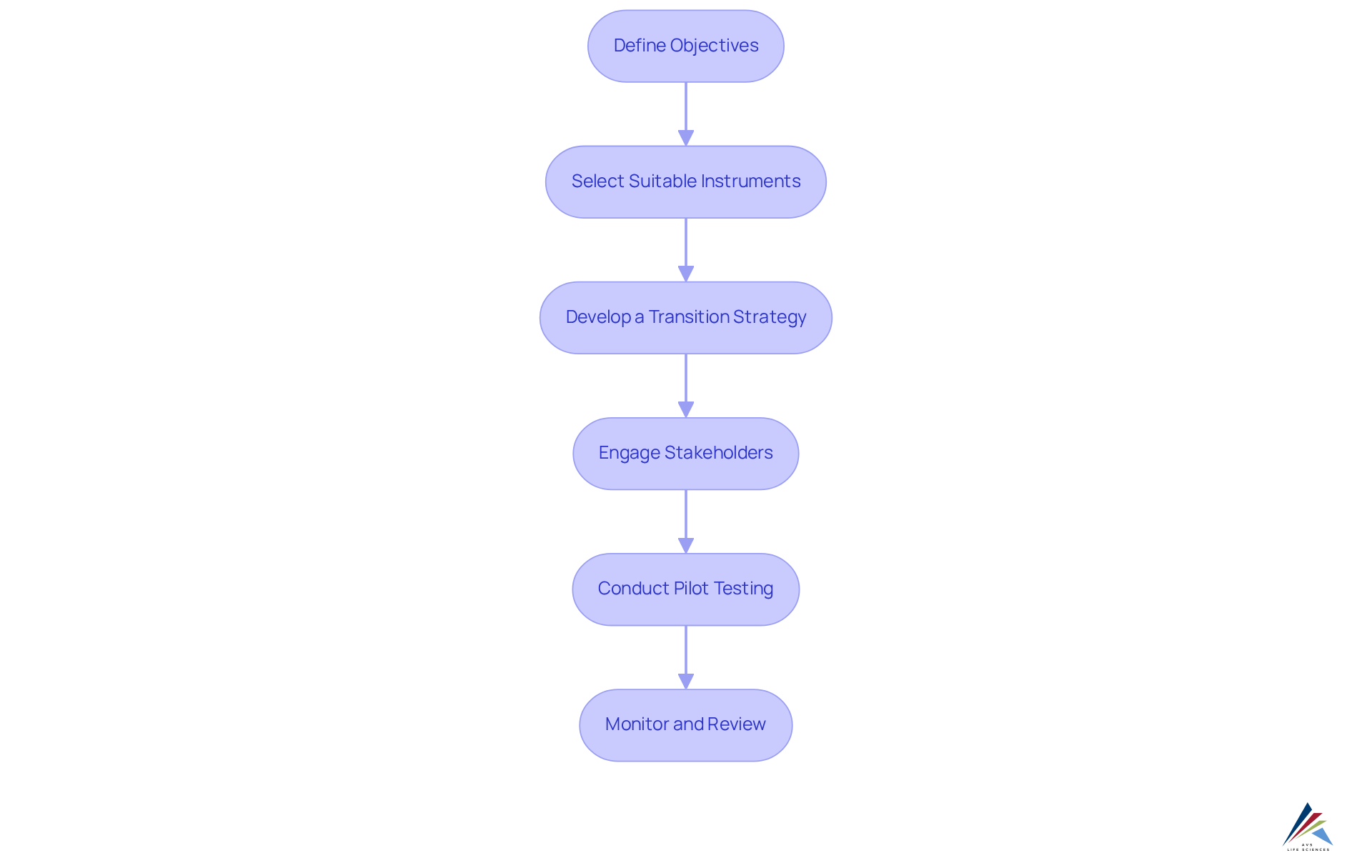
To implement effective digital validation strategies, organizations must adhere to these essential steps:
- Define Objectives: Clearly outline the goals of electronic verification, encompassing compliance requirements, efficiency improvements, and risk management. This alignment ensures that the verification process supports organizational objectives while adhering to legal standards.
- Select Suitable Instruments: Choose electronic assessment tools tailored to the organization’s specific needs. Crucial factors include scalability, user-friendliness, and compliance with regulatory standards, particularly as 66% of organizations anticipate a rise in the adoption of these technologies.
- Develop a Transition Strategy: Formulate a comprehensive plan for transitioning from traditional assessment methods to electronic processes. This strategy should detail timelines, resource allocation, and training requirements for personnel, facilitating a seamless shift to electronic approval.
- Engage Stakeholders: Involve key stakeholders from various departments, including IT, quality assurance, and regulatory affairs. This collaboration fosters a holistic approach to online verification, ensuring all perspectives are considered.
- Conduct Pilot Testing: Prior to full-scale implementation, carry out pilot tests to uncover potential issues and refine processes. This phase allows organizations to make adjustments based on real-world insights, enhancing the efficacy of the online assessment approach.
- Monitor and Review: Post-implementation, continuously monitor electronic verification processes to guarantee compliance and effectiveness. Regular reviews and updates are vital to adapt to changing regulations and technological advancements, fostering a culture of continuous improvement.
By following these steps, organizations can effectively execute electronic verification strategies that enhance compliance and operational efficiency. This proactive approach not only streamlines product development but also equips companies to respond swiftly to regulatory changes, ultimately leading to improved outcomes in regulatory approval.
Ensure Continuous Training and Support for Compliance
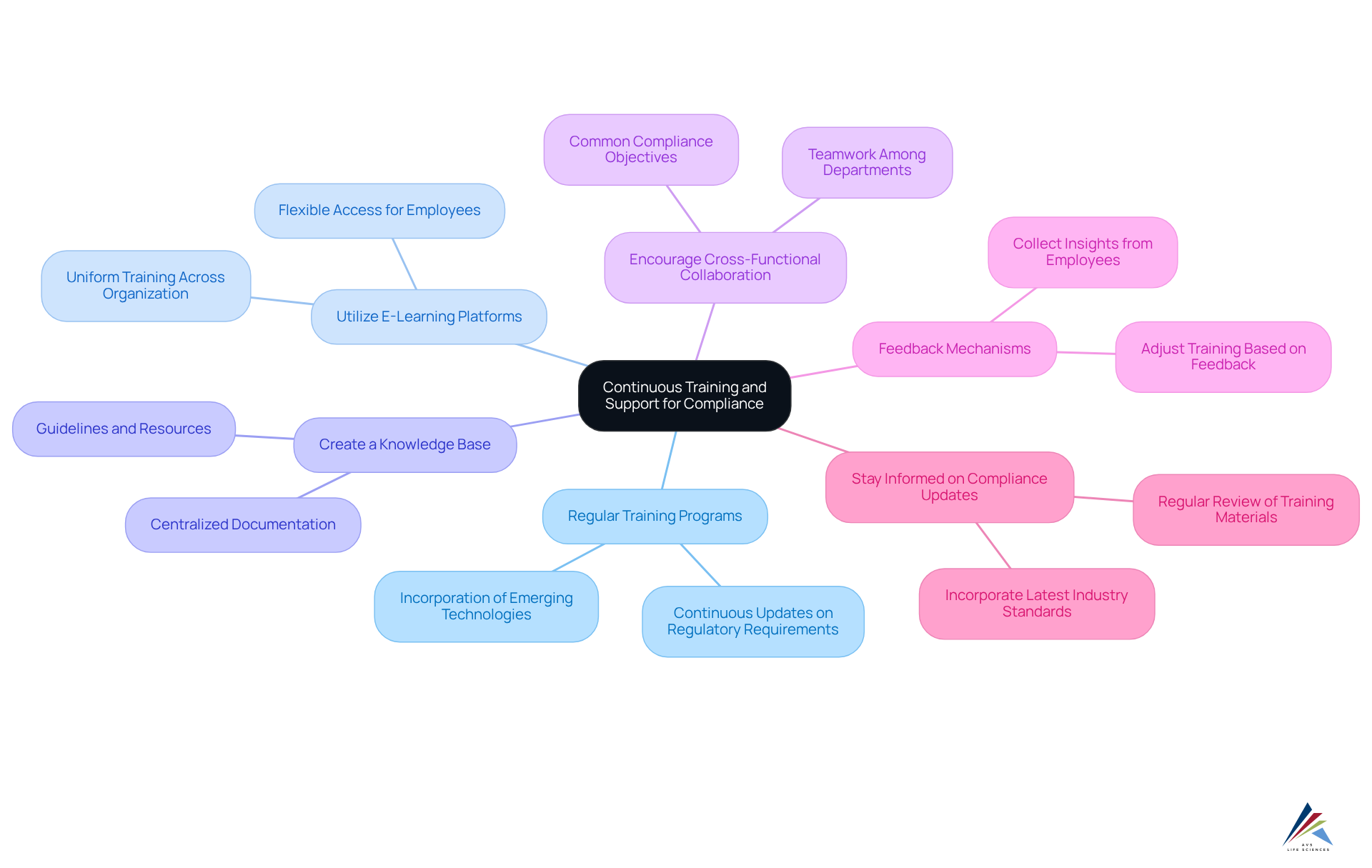
To maintain robust compliance in digital validation, organizations must adopt best practices that effectively address compliance challenges:
- Regular Training Programs: Implement continuous training sessions specifically designed for employees involved in digital verification. These sessions must include updates on regulatory requirements, emerging technologies, and best practices, ensuring that all staff remain well-informed.
- Utilize E-Learning Platforms: Leverage to provide flexible training options that employees can access at their convenience. This approach promotes uniform training across the organization, accommodating diverse learning paces and schedules.
- Create a Knowledge Base: Develop a centralized knowledge base containing documentation, guidelines, and resources pertinent to digital validation. This repository serves as a vital reference for employees, fostering consistency in practices and decision-making.
- Encourage Cross-Functional Collaboration: Promote teamwork among departments such as IT, quality assurance, and compliance affairs. This alignment ensures that all teams work towards common compliance objectives, enhancing overall effectiveness.
- Feedback Mechanisms: Establish feedback channels to collect insights from employees regarding the training programs and support offered. This input is essential for ongoing enhancement, enabling organizations to adjust training to meet evolving requirements.
- Stay Informed on Compliance Updates: Regularly review and revise training materials to reflect the latest compliance changes and industry standards. Keeping employees informed about current compliance requirements is essential for maintaining a compliant environment.
By prioritizing these practices, organizations can strengthen their compliance frameworks, mitigate risks, and ensure that the digital validation implementation processes are effective and aligned with regulatory expectations.
Conclusion
Implementing digital validation in the pharmaceutical industry represents not just a trend, but an essential step towards ensuring compliance and achieving operational excellence. By embracing electronic verification, organizations can significantly enhance their capacity to maintain data integrity and streamline processes, ultimately leading to superior regulatory outcomes.
This article outlines critical strategies for effective digital validation, including:
- Defining clear objectives
- Selecting appropriate tools
- Developing a robust transition plan
Engaging stakeholders across departments and conducting thorough pilot tests are vital for ensuring a smooth implementation. Furthermore, continuous monitoring and training are pivotal in adapting to the evolving regulatory landscape, equipping employees with the latest knowledge and skills.
As the pharmaceutical sector advances towards a more digital future, the significance of digital validation cannot be overstated. Organizations are urged to prioritize these strategies and cultivate a culture of continuous improvement. By doing so, they not only enhance compliance but also position themselves to respond swiftly to regulatory changes, ultimately driving better outcomes in product development and approval processes. Embracing digital validation transcends merely keeping pace with industry changes; it embodies leading the way towards a more efficient and compliant pharmaceutical landscape.
Frequently Asked Questions
What is digital validation in pharmaceutical compliance?
Digital validation refers to the electronic verification of systems and processes within the pharmaceutical sector to ensure they function correctly and adhere to industry standards, guaranteeing data integrity, accuracy, and reliability.
What are the key components of digital validation?
The key components of digital validation include risk assessment, thorough documentation, and strict adherence to regulatory guidelines such as 21 CFR Part 11 and EU GMP Annex 11.
How does electronic verification improve compliance and operational efficiency?
Electronic verification enhances compliance and operational efficiency by transitioning organizations from traditional validation methods to more efficient digital solutions, which can lead to significant improvements in assessment procedures and faster product development cycles.
What impact did a pharmaceutical firm experience after adopting an electronic verification system?
A pharmaceutical firm that adopted an electronic verification system experienced a 30% reduction in verification time, which accelerated product development cycles and improved audit outcomes.
What role does AI-assisted verification play in the pharmaceutical sector?
AI-assisted verification can preserve entire batches and significantly reduce deviation investigation time, showcasing the efficiency gains from using advanced verification tools.
What challenges does the pharmaceutical sector face regarding electronic verification?
The sector faces challenges such as a shortage of human resources for verification tasks and the need for comprehensive electronic audit trails to demonstrate data integrity, as expected by regulatory bodies like the FDA and EMA.
What solutions does AVS Life Sciences offer for electronic verification challenges?
AVS Life Sciences provides comprehensive solutions that include strategies for managing data integrity deviations, conducting investigations, and implementing Corrective and Preventive Action (CAPA) processes to help organizations navigate electronic verification complexities.
