3 Key Research Solutions for Enhanced Pharmaceutical Compliance

Overview
The article examines three pivotal research solutions designed to enhance pharmaceutical compliance, underscoring the significance of advanced data analytics, effective investigation strategies, and continuous evaluation.
By illustrating how organizations that utilize these solutions report notable improvements in regulatory outcomes, it highlights reduced compliance-related costs and enhanced operational efficiency.
This, in turn, mitigates the financial risks associated with non-adherence, emphasizing the necessity for proactive engagement with compliance solutions.
Introduction
Navigating the intricate landscape of pharmaceutical compliance presents significant challenges, particularly as regulatory requirements evolve rapidly. Research solutions have emerged as essential resources, equipping organizations with the insights and data necessary to identify compliance gaps and enhance operational effectiveness. Given the substantial financial penalties associated with non-compliance, a critical question arises: how can companies effectively integrate these research methodologies to not only meet but exceed regulatory expectations? By leveraging these insights, organizations can not only safeguard against penalties but also position themselves as leaders in compliance excellence.
Understand the Role of Research Solutions in Compliance
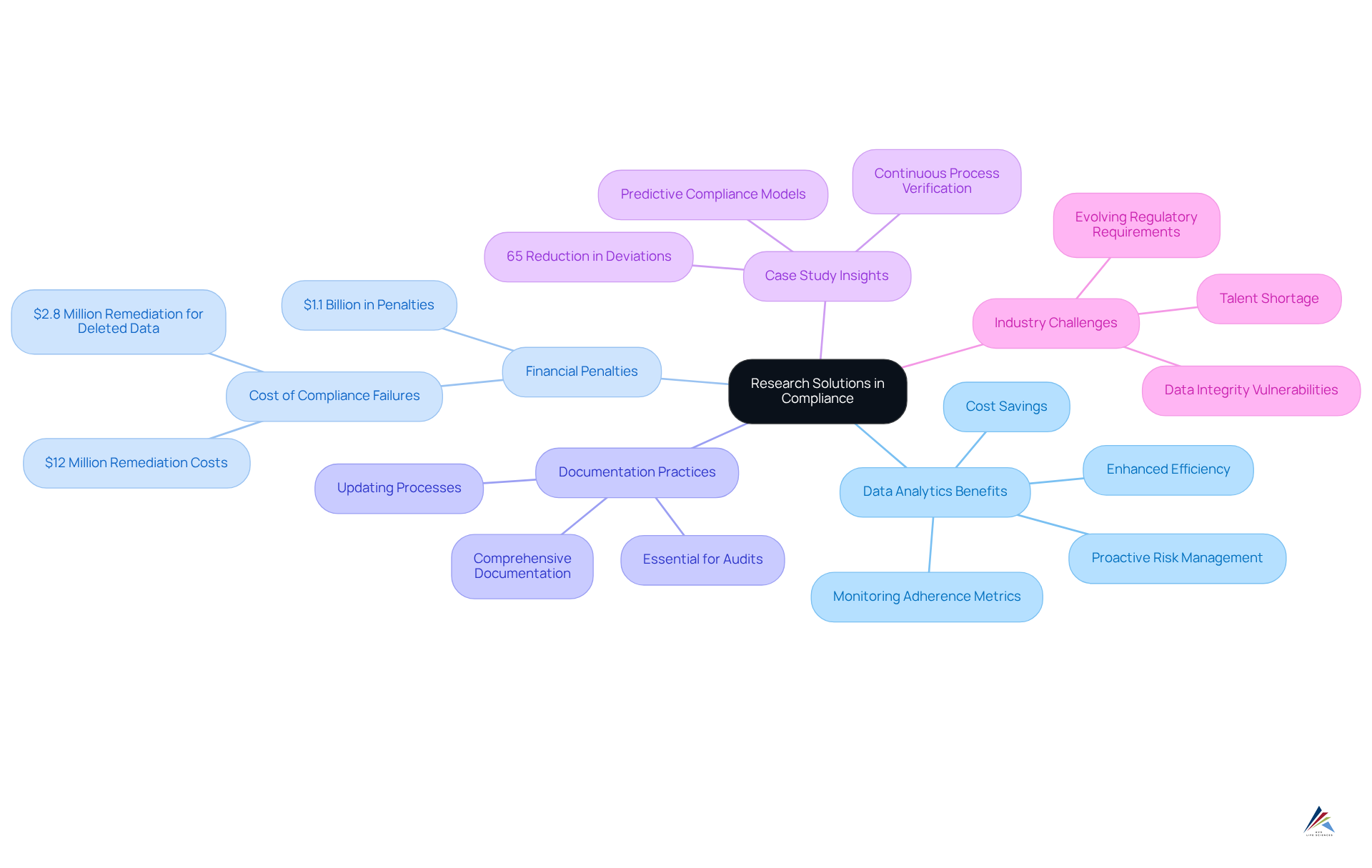
Ensuring adherence in the pharmaceutical sector is essential, and research solutions deliver critical data and insights that identify potential gaps and inform strategic decision-making. By leveraging advanced data analytics, organizations can effectively monitor adherence metrics, facilitating proactive adjustments to processes that align with evolving regulatory requirements.
Companies that have integrated data analytics into their regulatory operations report enhanced efficiency and a significant reduction in audit findings. Notably, regulatory failures in the pharmaceutical sector have resulted in $1.1 billion in penalties over the past five years, underscoring the and the importance of robust data analytics in mitigating such risks.
Moreover, these research solutions strengthen the development of comprehensive documentation practices, which are essential for successful audits and regulatory submissions. Entities that prioritize investigation in their adherence strategies often experience superior outcomes, including a marked decrease in regulation-related costs and heightened operational effectiveness.
A case study highlighted that a mid-sized injectable manufacturer reduced data-related deviations by 65% through expert collaboration, demonstrating the transformative potential of data analytics in regulatory management. Furthermore, 72% of quality specialists in the drug industry report challenges in maintaining compliance with shifting regulations, emphasizing the necessity of integrating analytical solutions into adherence strategies.
As the pharmaceutical landscape continues to evolve, embedding research solutions into adherence strategies will be essential for maintaining high standards and achieving regulatory success.
Implement Effective Research Strategies for Compliance
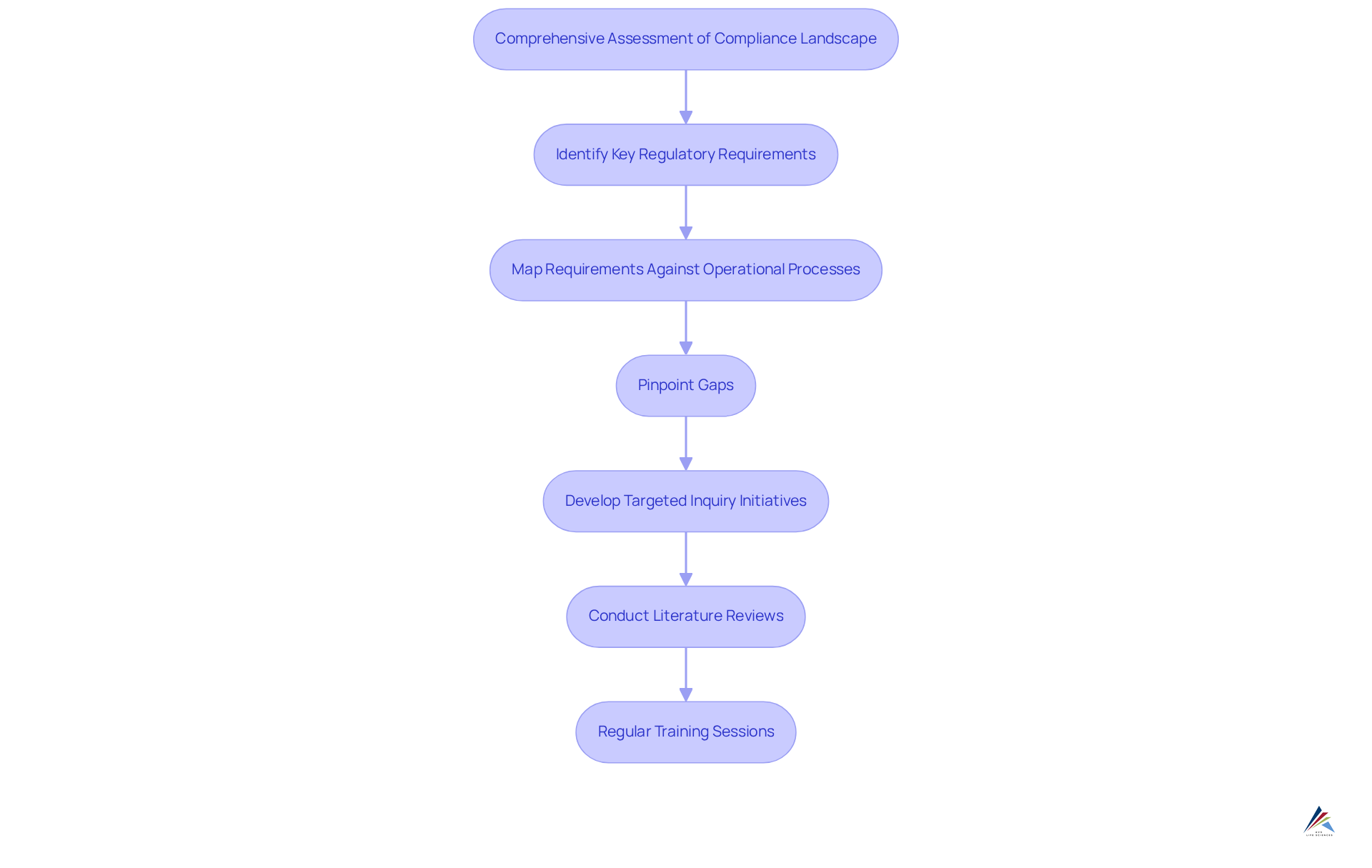
To implement , organizations must commence with a comprehensive assessment of their current compliance landscape. This critical process entails identifying key regulatory requirements, including those established by the FDA and EMA, and systematically mapping them against existing operational processes. By pinpointing gaps, organizations can develop targeted inquiry initiatives to rectify these deficiencies. For instance, conducting literature reviews and benchmarking against industry best practices can yield valuable insights into effective regulatory measures.
Cooperation between investigation groups and regulatory divisions is paramount for enhancing knowledge exchange and ensuring that study results are seamlessly integrated into regulatory procedures. Regular training sessions on research solutions empower staff to apply these methodologies effectively, fostering a culture of adherence.
Entities that have embraced these collaborative methods have reported significant improvements in their research solutions for regulatory outcomes. For example, companies leveraging advanced regulatory analytics have experienced a 41% enhancement in regulatory inspection results and a 30% decrease in compliance-related costs. By prioritizing these best practices, medical organizations can fortify their regulatory posture and promote operational excellence.
Evaluate and Adapt Research Solutions for Ongoing Compliance
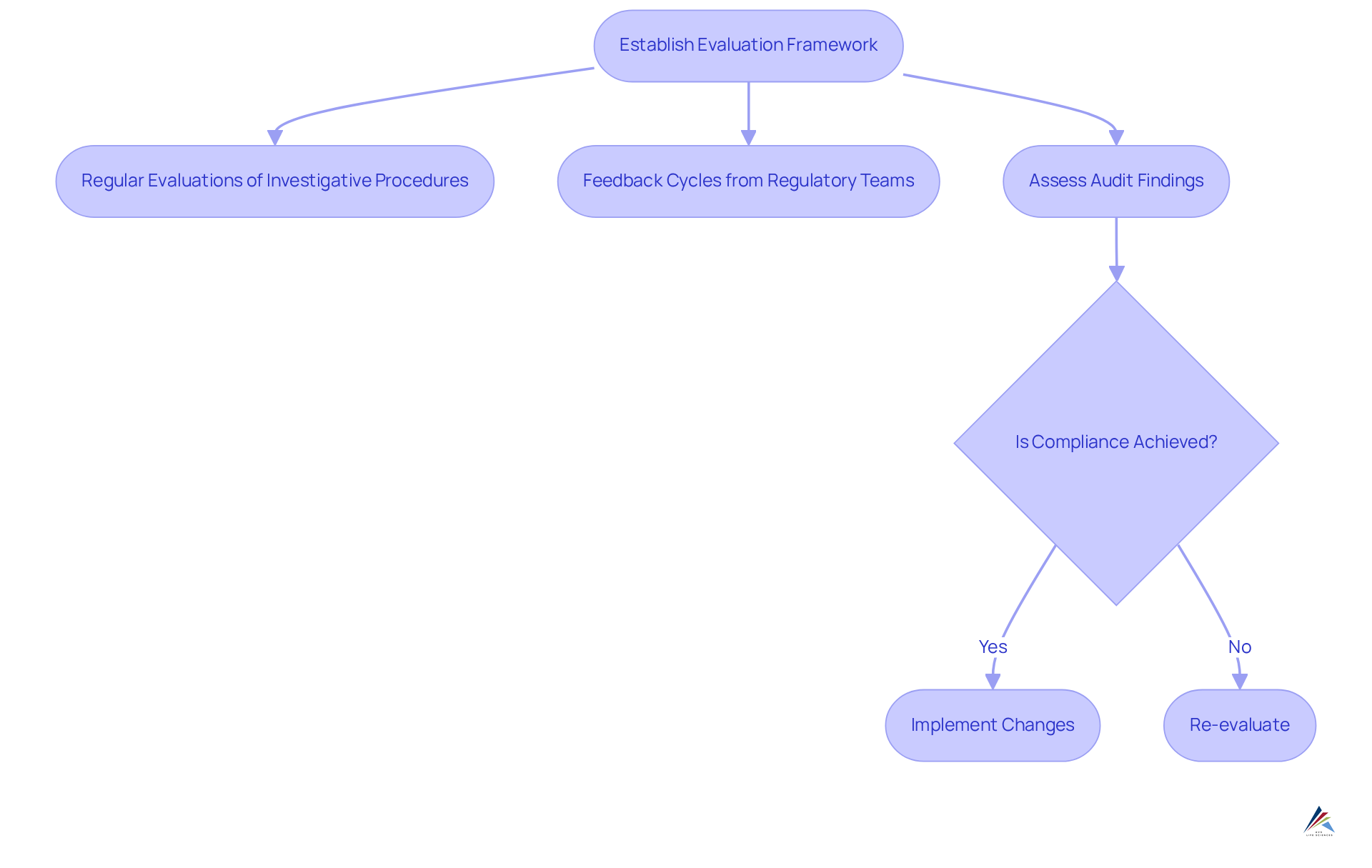
Continuous assessment and adjustment of investigative solutions are crucial for maintaining adherence in the pharmaceutical sector. Organizations must establish a framework for routinely evaluating their investigative methods and adherence outcomes. This framework should include:
- Regular evaluations of investigative procedures
- Feedback cycles from regulatory teams
- Assessments of audit findings to identify areas for enhancement
Furthermore, staying informed about changes in compliance requirements and industry standards is essential for timely adjustments. For instance, when new directives are issued by regulatory agencies, organizations must swiftly assess their existing research solutions and implement necessary changes to ensure continued compliance. Engaging with external specialists and participating in industry discussions can provide valuable insights into emerging trends and best practices, further enhancing an organization’s regulatory position. Notably, 67% of pharmaceutical producers have adopted or are in the process of adopting real-time regulatory monitoring systems, underscoring the trend towards external involvement and its benefits. Organizations that take a proactive approach to compliance management—such as those highlighted in the Cleveland Clinic's predictive compliance program, which achieved a 50% reduction in regulatory citations—report substantial improvements in operational efficiency and regulatory standing. This highlights the critical importance of .
Conclusion
Embedding research solutions into pharmaceutical compliance strategies is crucial for navigating the complexities of regulatory requirements. These solutions provide valuable insights and enhance operational efficiency, enabling organizations to proactively address gaps and ensure adherence to evolving standards.
The article highlights several key arguments, including:
- The significant financial implications of non-compliance
- The transformative potential of data analytics
- The necessity of continuous evaluation and adaptation of research methodologies
By integrating effective investigation strategies and fostering collaboration between teams, pharmaceutical companies can achieve superior outcomes and reduce compliance-related costs. The case studies presented demonstrate that organizations adopting these best practices experience marked improvements in regulatory inspection results and operational effectiveness.
Ultimately, the integration of research solutions is not merely a compliance measure but a strategic imperative for pharmaceutical organizations aiming to thrive in a competitive landscape. By prioritizing adaptability and continuous improvement in their compliance processes, companies can mitigate risks and enhance their overall regulatory standing. Embracing these practices will lead to a more resilient, efficient, and compliant pharmaceutical sector, positioning organizations for long-term success in an ever-evolving industry.
Frequently Asked Questions
What is the role of research solutions in compliance within the pharmaceutical sector?
Research solutions play a crucial role in ensuring adherence by providing critical data and insights that identify potential gaps and inform strategic decision-making.
How do advanced data analytics benefit organizations in the pharmaceutical industry?
Advanced data analytics enable organizations to effectively monitor adherence metrics, allowing for proactive adjustments to processes that align with evolving regulatory requirements.
What are the consequences of regulatory failures in the pharmaceutical sector?
Regulatory failures can lead to significant financial repercussions, with penalties amounting to $1.1 billion in the past five years due to non-adherence.
How do companies benefit from integrating data analytics into their regulatory operations?
Companies that integrate data analytics report enhanced efficiency and a significant reduction in audit findings.
Why are comprehensive documentation practices important in the pharmaceutical industry?
Comprehensive documentation practices are essential for successful audits and regulatory submissions, helping to ensure compliance.
What outcomes do entities experience when they prioritize investigation in their adherence strategies?
Entities that prioritize investigation often see superior outcomes, including a decrease in regulation-related costs and increased operational effectiveness.
Can you provide an example of how data analytics has transformed regulatory management?
A case study showed that a mid-sized injectable manufacturer reduced data-related deviations by 65% through expert collaboration, highlighting the transformative potential of data analytics.
What challenges do quality specialists in the drug industry face regarding compliance?
72% of quality specialists report challenges in maintaining compliance with shifting regulations, indicating the need for integrating analytical solutions into adherence strategies.
Why is it important to embed research solutions into adherence strategies as the pharmaceutical landscape evolves?
Embedding research solutions into adherence strategies is essential for maintaining high standards and achieving regulatory success in a continually changing environment.
