3 Key Practices for Being Inspection Ready in Pharma
Overview
To achieve inspection readiness in the pharmaceutical industry, organizations must prioritize the establishment of robust Quality Management Systems (QMS) along with instilling a quality mindset in all departments. This involves implementing effective training and documentation procedures and adopting proactive compliance strategies. These practices not only enhance compliance to current regulations but also improve operational efficiency. Daily manufacturing/laboratory area walk throughs by management internal audits and mock inspections along with measurement of Key Performance Indicators (KPI) provide critical information on areas of focus for continuous improvement. By embracing these strategies, organizations can ensure they are not just compliant but also operate in an inspection ready state to be positioned for success in a competitive landscape.
Introduction
In the highly regulated pharmaceutical industry, operating in an inspection ready manner is critical to maintaining a state of control. Organizations that prioritize effective quality management systems, robust documentation and training programs, and proactive compliance strategies not only ensure adherence to regulatory standards but also enhance their operational efficiency and reputation. However, with evolving regulations and increasing scrutiny, companies face significant challenges in maintaining their facilities in a state of inspection readiness.
How can you avoid the common pitfalls? This article explores three key practices that can empower pharmaceutical organizations to maintain compliance and foster a culture of continuous improvement.
Establish Robust Quality Management Systems
Organizations must establish a robust Quality Management System (QMS) that encompasses all aspects of their operations to be inspection ready. This involves outlining standards, writing effective and easy to follow policies and procedures, establishing clear objectives, and assigning responsibilities to the appropriate staff. Key components of an effective QMS include:
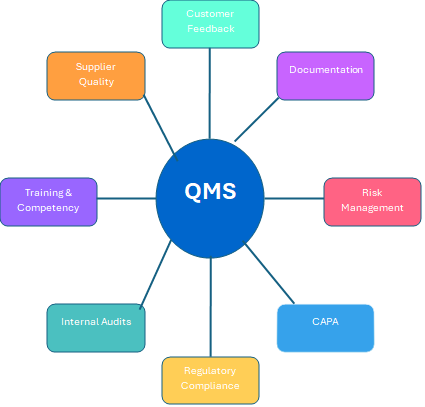
Comprehensive Documentation: Industry leaders highlight that effective document management is essential to a successful QMS, as it directly affects adherence and operational efficiency. By prioritizing these components, organizations can establish a culture of quality that fulfills compliance expectations along with being inspection ready.
Every batch record, deviation, and Corrective and Preventive Action (CAPA) should be meticulously documented according to Good Documentation Practices. This documentation should tell a coherent story of your quality system, showing not just what happened, but why decisions were made and how they connect to patient safety and product quality.
Regular Audits and Reviews: Conducting regular internal audits and management reviews ensures that the QMS is functioning effectively and helps identify areas for improvement before an external inspection.
Continuous Training and Personnel Readiness:
Your personnel are as important as your documentation. Ensuring that your team is well-trained and inspection-ready involves:
Ongoing Training Programs: Implementing continuous training programs that focus on building a deep understanding of quality principles and their application. This helps personnel articulate their roles, explain their decisions, and demonstrate their understanding during inspections
Simulation Exercises: Conducting mock inspections and simulation exercises to prepare staff for real inspection scenarios. This helps in building confidence and ensuring that everyone knows their responsibilities during an actual inspection
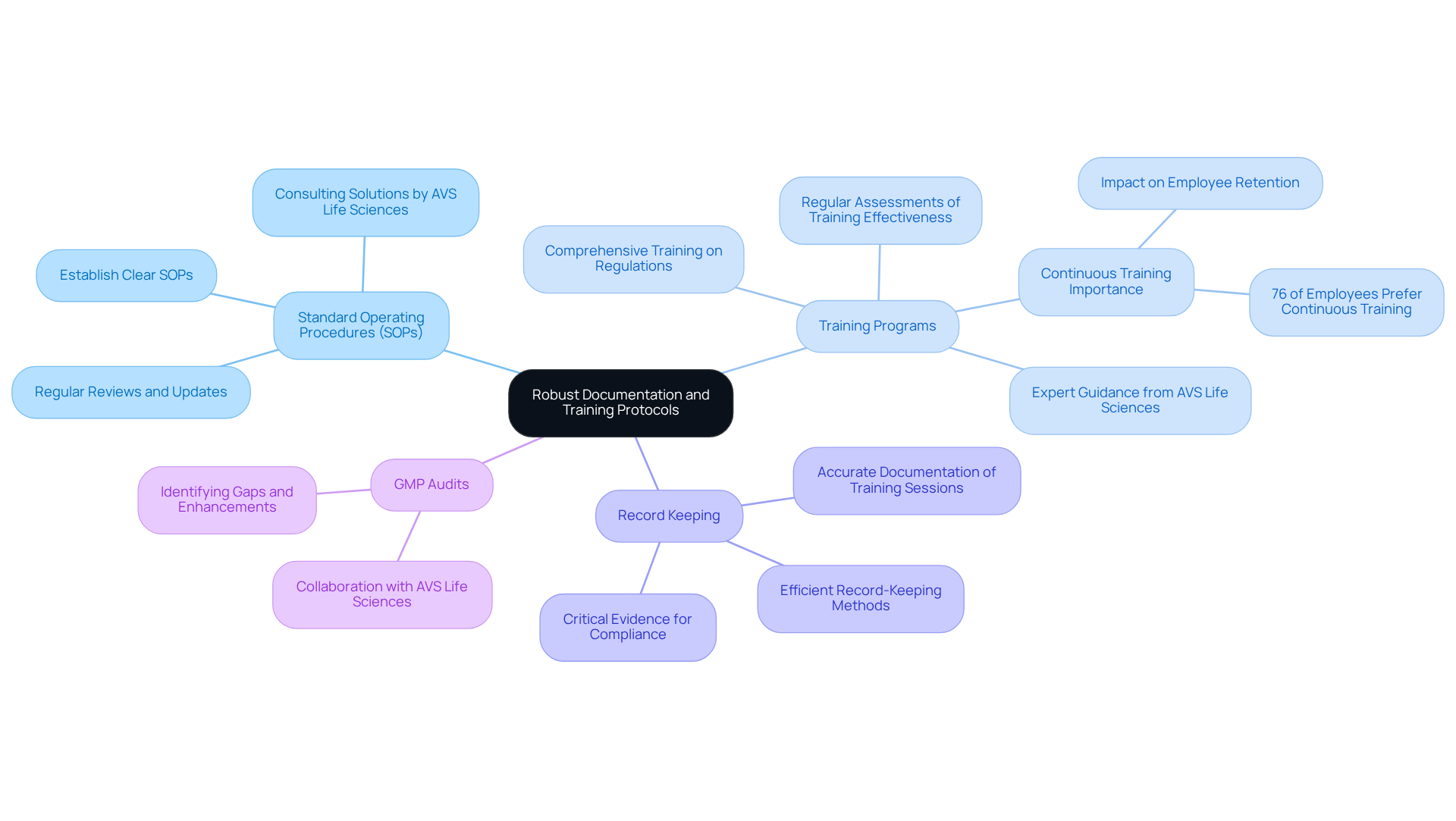
Implement Robust Documentation and Training Protocols
In the pharmaceutical industry, being inspection-ready and maintaining compliance fundamentally relies on robust documentation and effective training protocols. Organizations must prioritize the following key areas:
By concentrating on these aspects, entities can cultivate a skilled workforce prepared to uphold quality standards and navigate the complexities of regulations, with AVS Life Sciences as a reliable ally in their journey.
Proactive Compliance Strategies
To ensure inspection readiness, organizations must embrace proactive compliance and continuous improvement strategies, which include:
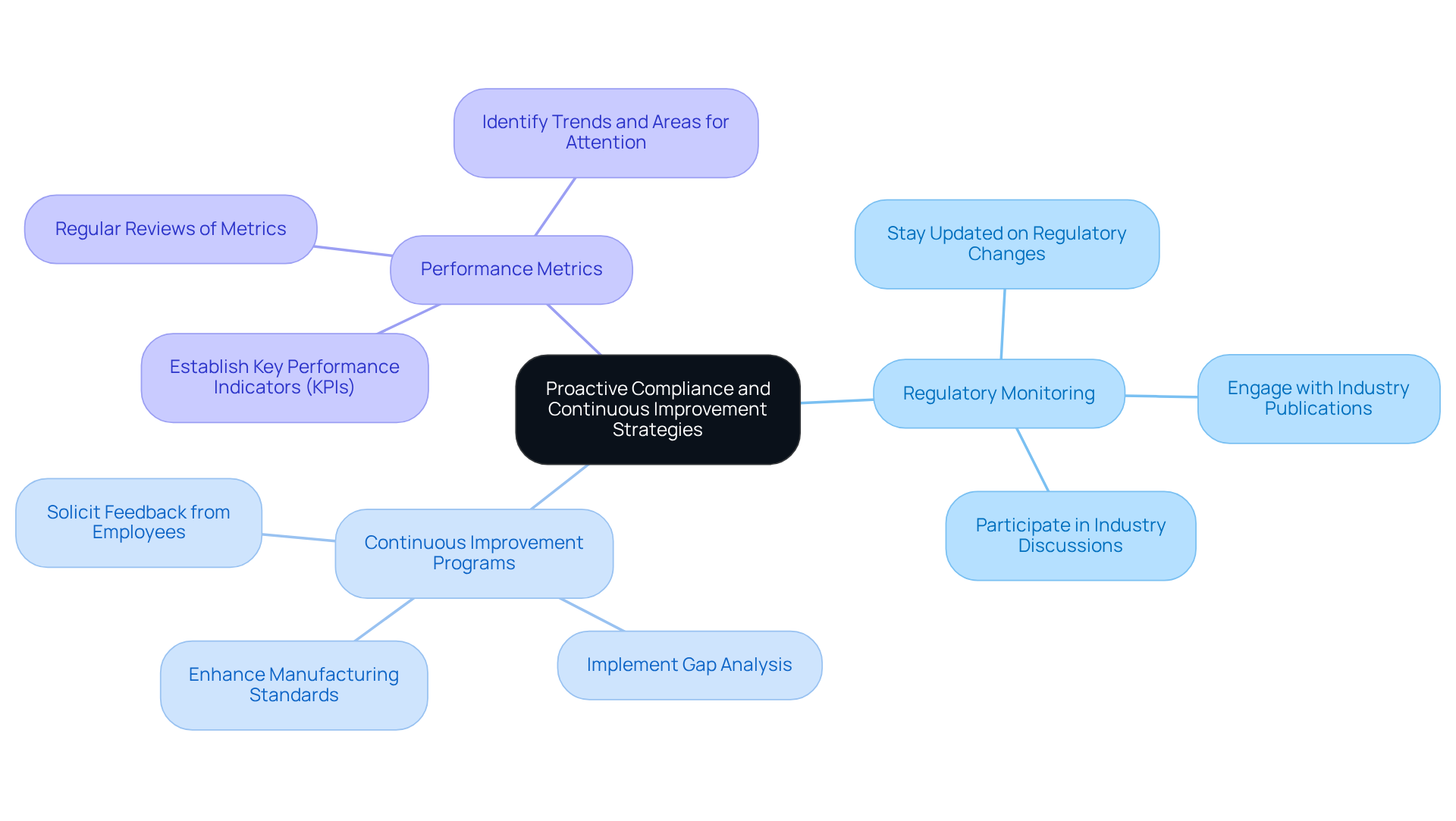
Regular Monitoring and Reporting: Implementing systems for regular monitoring and reporting of compliance metrics. This helps in identifying potential issues early and addressing them promptly
Effective CAPA Management: Ensuring that your CAPA system demonstrates thorough investigation, appropriate corrective actions, and verification of their effectiveness. This shows inspectors that you have robust problem management processes in place
By adopting a proactive approach, companies become inspection ready and also cultivate a culture of continuous improvement that enhances overall quality management efforts. This commitment to continuous improvement is essential in navigating the complexities of the pharmaceutical sector, where regulatory adherence is critical. However, organizations should remain vigilant against common pitfalls, such as inadequate training, lack of employee engagement, and inadequate human resources, which can impede the successful execution of these strategies. Ultimately, the anticipated impact of these practices encompasses improved compliance, operational efficiency, and a strengthened reputation within the industry.
By focusing on these three key practices, pharmaceutical companies can maintain a state of constant readiness, ensuring that they are always prepared for regulatory inspections.
Conclusion
Achieving inspection readiness in the pharmaceutical industry demands that organizations prioritize the establishment of comprehensive quality management systems, embrace a quality mindset, provide robust training and documentation that conforms to Good Documentation Practices, and proactive compliance strategies that support continuous improvement. The core of being prepared for inspections is rooted in fostering a culture of quality and continuous improvement, which not only meets regulatory requirements but also enhances operational efficiency and stakeholder confidence.
This article highlights three critical practices. Each of these practices plays a significant role in creating an environment where compliance is ingrained in daily operations, thereby minimizing risks and enhancing overall performance.
Ultimately, organizations in the pharmaceutical sector must recognize that being inspection-ready is not merely a box to check; it is a vital component of operational excellence. By committing to these key practices, companies can navigate the complexities of regulatory landscapes and position themselves as industry leaders dedicated to quality and innovation. Embracing these strategies will lead to improved compliance, operational efficiency, and a stronger reputation within the industry, making it imperative for all stakeholders to prioritize inspection readiness as a fundamental aspect of their operational framework.
Frequently Asked Questions
What is a Quality Management System (QMS)?
A Quality Management System (QMS) is a structured system that outlines standards, policies, objectives, and responsibilities to ensure that an organization operates effectively and is inspection ready.
What are the key components of an effective QMS?
The key components of an effective QMS include Document Control, Risk Management, and Internal Audits.
Why is Document Control important in a QMS?
Document Control is important because it ensures that all documents are current, accessible, and properly reviewed, which is essential for compliance with regulatory standards.
How does effective Document Control impact customer grievances?
Firms that invest in quality management practices experience decreased customer complaints, demonstrating the positive impact of efficient Document Control on overall adherence.
What role does Risk Management play in a QMS?
Risk Management involves identifying potential risks in processes and establishing strategies to mitigate them, which helps organizations adapt to operational changes and improve their regulatory standing.
How do internal audits contribute to a QMS?
Internal audits evaluate adherence to established procedures and identify areas for improvement, promoting a culture of excellence and ensuring organizations are inspection ready for external regulatory and sponsor inspections.
How can AVS Life Sciences assist organizations in establishing a QMS?
AVS Life Sciences provides customized solutions and expert guidance to help organizations establish strong document control systems, develop risk management frameworks, and perform internal audits to enhance adherence and operational efficiency.
Reference: FDA Inspection Readiness in 2025: A Complete Guide & Checklist
