3 Key Differences Between FDA Clearance vs Approval You Must Know

Overview
The three key differences between FDA clearance and approval encompass the regulatory processes, timeframes for acquisition, and compliance requirements. Notably, clearance is typically expedited, necessitating the demonstration of substantial equivalence to existing products via a 510(k) submission. In contrast, approval demands extensive clinical data and a rigorous evaluation process, reflecting the varying levels of risk and scrutiny associated with medical devices and drugs. Understanding these distinctions is crucial for navigating the compliance landscape effectively.
Introduction
Navigating the complex landscape of medical device regulation can be daunting, particularly when grasping the critical distinctions between FDA clearance and approval. While both pathways are essential for bringing products to market, they serve distinct purposes and involve varying degrees of scrutiny. This article delves into the key differences between these two regulatory processes, revealing how the choice between clearance and approval can significantly impact market entry strategies and compliance obligations. Manufacturers face numerous challenges when deciding which route to pursue—understanding these differences can lead to more informed decisions.
Define FDA Clearance and Approval
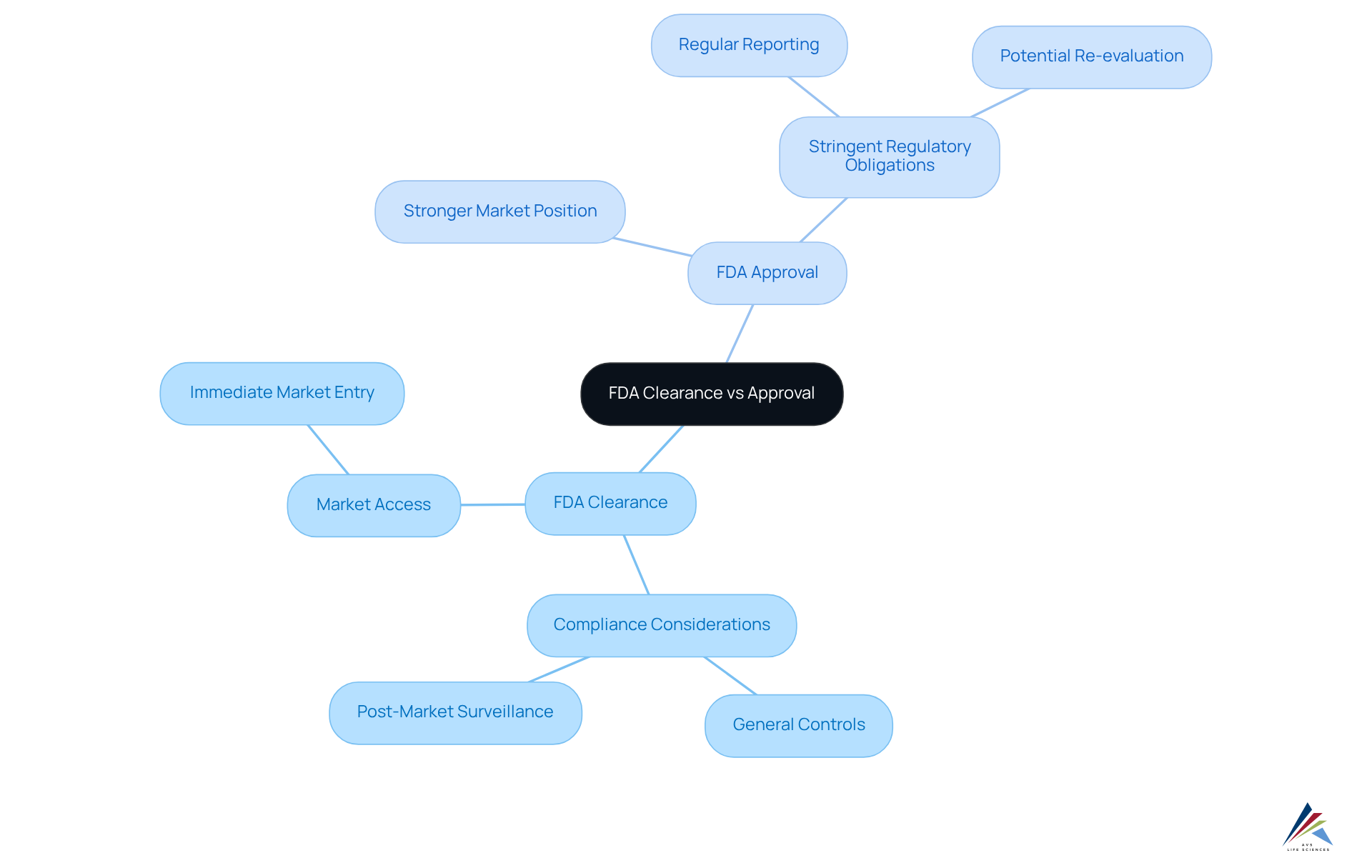
FDA approval represents the process through which the FDA permits a medical instrument to be marketed, contingent upon establishing significant equivalence to an already legally marketed product. This is primarily achieved via the 510(k) submission method, typically characterized by less stringent requirements compared to the validation procedure. Conversely, FDA approval serves as a more rigorous pathway, designated for new drugs and certain high-risk medical devices. This pathway necessitates comprehensive clinical data to substantiate both safety and efficacy. Approval is granted solely after the FDA conducts a thorough review and validation of the submitted data, ensuring that the benefits of the product significantly outweigh its risks for the intended use.
In 2021, approximately 85 percent of 510(k) applications received a Substantially Equivalent decision, thereby facilitating their market entry. However, nearly 32 percent of submissions did not pass the initial acceptance for review check, underscoring the complexities inherent in the clearance system. The average timeframe to receive a decision on 510(k) applications has been reported to be around five months, often extended due to requests for additional information from the FDA. This duration starkly contrasts with the more stringent evaluation method, which can require significantly longer and demands thorough proof of a product's safety and effectiveness, particularly for Class III items that present greater risks.
Understanding these distinctions is crucial for effectively navigating the regulatory landscape, as the choice between FDA clearance vs approval can profoundly impact a product's market entry strategy. AVS Life Sciences offers comprehensive regulatory and quality solutions across biopharmaceuticals, medical instruments, and nutraceuticals, empowering clients to adeptly navigate the complexities of FDA clearance and authorization processes. Our expertise in these domains positions us as your trusted partner in achieving compliance and facilitating successful market entry.

Contrast Regulatory Processes: Clearance vs. Approval
The regulatory pathways for FDA clearance vs approval are fundamentally different, presenting distinct compliance challenges. Clearance is achieved through a 510(k) submission, where manufacturers must demonstrate that their device is substantially equivalent to an existing one. This procedure can be finalized in as little as 90 days, although the typical duration for a decision is approximately 147 days.
In contrast, the authorization procedure, particularly for new medications, entails a New Drug Application (NDA) or Premarket Approval (PMA) submission, which requires extensive clinical trial data and may take several years. This thorough evaluation system generally comprises multiple stages of clinical trials to meticulously assess safety and effectiveness.
While clearance is typically quicker and cheaper, appealing to producers of lower-risk devices, the validation process is more rigorous, indicating the greater stakes associated with high-risk medical products. For instance, roughly 75-80% of medications receive authorization after their initial review cycle; however, the median duration from NDA submission to FDA endorsement is around 12 months for standard evaluations.
Understanding the differences in FDA clearance vs approval is crucial for navigating the regulatory landscape effectively and ensuring compliance within this complex framework.

Evaluate Market Impact and Compliance Considerations
The distinction of FDA clearance vs approval carries substantial market implications. Products that secure clearance can promptly enter the market, allowing companies to adeptly respond to emerging trends and consumer demands. Conversely, products requiring authorization often enjoy a stronger market position due to the rigorous testing and validation processes they undergo, which can significantly bolster consumer trust. Notably, nearly 90% of doctors believe that FDA endorsement signifies that the benefits of a product outweigh its risks, thereby enhancing the credibility of approved items in the eyes of consumers.
Compliance considerations further delineate these pathways. Cleared devices must adhere to general controls and may require post-market surveillance to monitor safety and effectiveness. In contrast, approved products encounter more stringent ongoing regulatory obligations, including regular reporting and potential re-evaluation, which can complicate compliance efforts. Companies must meticulously assess these factors when formulating their regulatory strategy, as the decision between FDA clearance vs approval can profoundly influence market access, compliance burdens, and ultimately, their competitive positioning within the industry.
Conclusion
Understanding the distinctions between FDA clearance and approval is essential for navigating the regulatory landscape of medical devices and pharmaceuticals. While both processes aim to ensure product safety and efficacy, they differ significantly in terms of requirements, timelines, and market implications. FDA clearance generally allows for a quicker entry into the market through the 510(k) pathway, whereas FDA approval demands a more rigorous evaluation process, particularly for new drugs and high-risk devices.
The article highlights key aspects of these regulatory pathways, including the submission processes, the average timelines for decisions, and the compliance obligations that accompany each route. Products that achieve clearance can swiftly respond to market trends, while those that secure approval may benefit from enhanced consumer trust due to the thorough testing they undergo. The complexities of each process necessitate careful consideration by manufacturers to align their strategies with their product's risk profile and market goals.
Ultimately, a clear grasp of FDA clearance versus approval is not just a regulatory requirement; it is a strategic advantage. Companies must assess their options thoughtfully to navigate compliance effectively and position themselves competitively in the marketplace. By making informed decisions, businesses can optimize their pathways to market, ensuring that they meet both regulatory standards and consumer expectations.
Frequently Asked Questions
What is FDA clearance?
FDA clearance refers to the process through which the FDA permits a medical instrument to be marketed, primarily through the 510(k) submission method, which establishes significant equivalence to an already legally marketed product.
What is FDA approval?
FDA approval is a more rigorous process designated for new drugs and certain high-risk medical devices, requiring comprehensive clinical data to demonstrate both safety and efficacy before the FDA grants permission to market the product.
What is the difference between FDA clearance and approval?
The key difference is that FDA clearance typically involves less stringent requirements and is based on significant equivalence to existing products, while FDA approval requires extensive clinical data and a thorough review process, particularly for high-risk items.
What percentage of 510(k) applications received a Substantially Equivalent decision in 2021?
Approximately 85 percent of 510(k) applications received a Substantially Equivalent decision in 2021, facilitating their market entry.
How many 510(k) submissions did not pass the initial acceptance for review check?
Nearly 32 percent of 510(k) submissions did not pass the initial acceptance for review check, highlighting the complexities of the clearance system.
What is the average timeframe to receive a decision on 510(k) applications?
The average timeframe to receive a decision on 510(k) applications is around five months, although this can be extended due to requests for additional information from the FDA.
How does the timeframe for FDA approval compare to that of FDA clearance?
The timeframe for FDA approval is generally longer than that for FDA clearance, as it involves a more stringent evaluation process requiring thorough proof of a product's safety and effectiveness, particularly for Class III items that present greater risks.
Why is it important to understand the distinctions between FDA clearance and approval?
Understanding these distinctions is crucial for effectively navigating the regulatory landscape, as the choice between FDA clearance and approval can significantly impact a product's market entry strategy.
