21 CFR 111: Essential Steps for Compliance Success

Overview
The article delineates critical steps for achieving compliance with 21 CFR 111, which governs Current Good Manufacturing Practices (cGMP) for dietary supplements. It emphasizes the importance of:
- Quality control
- Personnel training
- Thorough documentation
These steps are not merely procedural; they are essential for organizations striving to meet regulatory standards. Moreover, effective compliance measures significantly enhance product safety and bolster company reputation. Evidence suggests that organizations adopting robust compliance strategies can realize substantial cost savings and improved operational efficiencies. In conclusion, prioritizing compliance is not only a regulatory obligation but a strategic advantage in the competitive landscape of dietary supplements.
Introduction
Navigating the complexities of dietary supplement manufacturing requires a firm grasp of regulatory standards, particularly 21 CFR 111, which outlines essential Good Manufacturing Practices (cGMP). As the demand for dietary supplements continues to rise, so does the imperative for companies to ensure compliance, safeguarding both consumer health and brand integrity.
However, many organizations grapple with the intricacies of these regulations. This raises a critical question: how can they effectively implement compliance measures to not only meet but exceed industry standards?
This article delves into the critical steps and strategies necessary for achieving success under 21 CFR 111. By empowering manufacturers with the knowledge and tools to thrive in an increasingly scrutinized landscape, we aim to foster a culture of compliance that benefits both businesses and consumers alike.
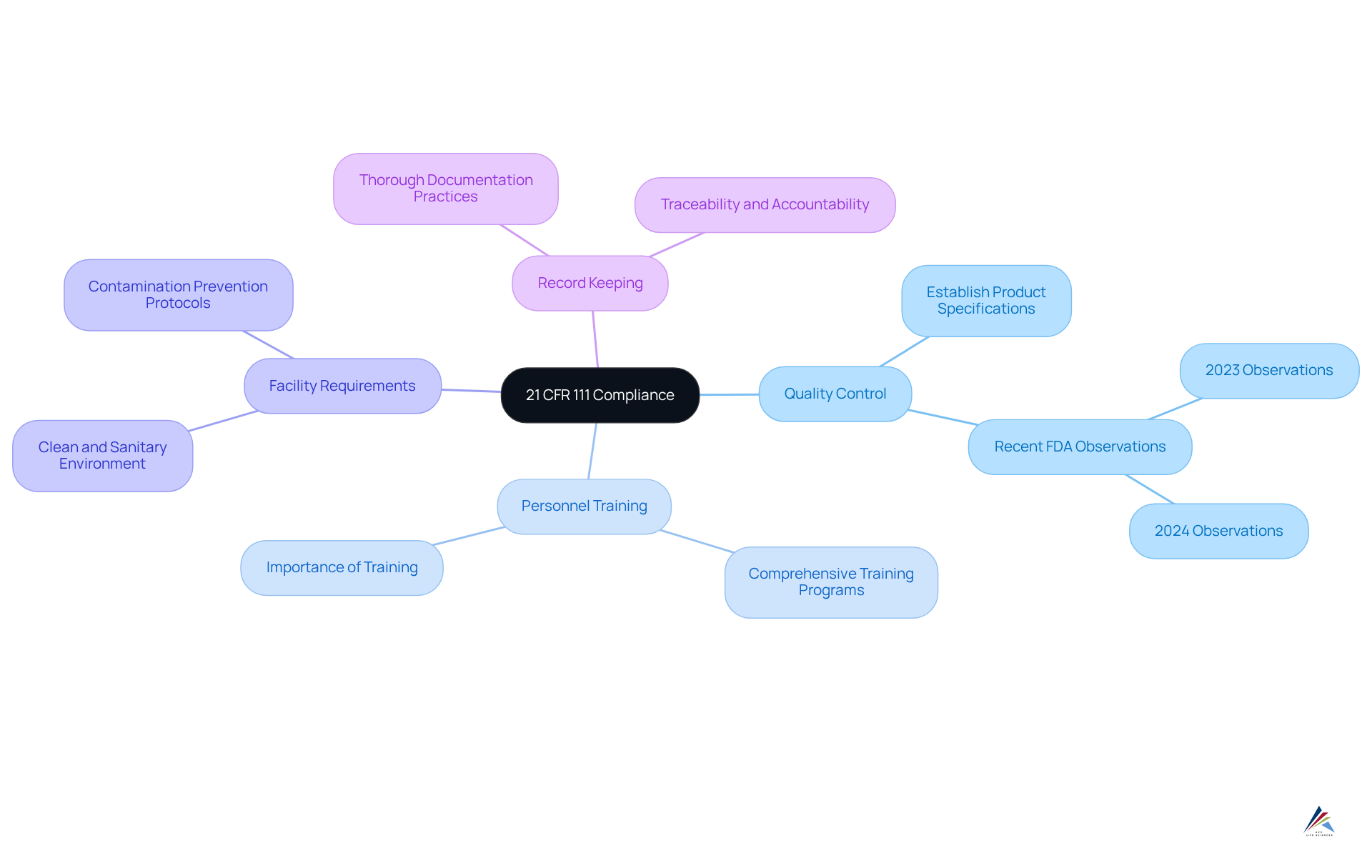
Understand 21 CFR 111: Key Principles and Requirements
21 CFR 111 delineates the Current Good Manufacturing Practices (cGMP) for dietary supplements, emphasizing several key principles essential for compliance:
- Quality Control: Establishing a robust quality control system is vital to ensure dietary supplements consistently meet safety and quality standards. Recent data indicates that the most frequent FDA observation in both 2023 and 2024 was the failure to establish product specifications for identity, purity, strength, and composition, underscoring the need for stringent quality measures.
- Personnel Training: Comprehensive training for all employees involved in manufacturing is crucial. This ensures that staff are well-versed in 21 CFR 111 requirements, which is fundamental for maintaining product integrity.
- Facility Requirements: A clean and sanitary manufacturing environment is essential to prevent contamination. Facilities must adhere to strict cleanliness protocols to safeguard product quality.
- Record Keeping: Thorough documentation of all processes—including production, testing, and distribution—is necessary for traceability and accountability. This practice not only aids in but also improves operational transparency.
The significance of quality control in dietary supplements cannot be overstated, as it directly impacts consumer safety and company reputation. Non-compliance with 21 CFR 111 can lead to severe legal repercussions, including the FDA's ability to seize drugs deemed 'adulterated' due to cGMP violations and damage to brand trust. As the industry advances, effective application of cGMP practices in accordance with 21 CFR 111 is progressively acknowledged as a characteristic of trustworthy dietary supplement producers, with numerous firms indicating enhanced adherence rates and operational efficiencies. Emphasizing these principles is essential for navigating the complexities of regulatory requirements and ensuring the delivery of safe, effective products. As Steve Mister, President & CEO, stated, "Dietary supplements have become an essential part of many Americans’ health routines," emphasizing the vital role of adherence in maintaining consumer trust.
Implement Compliance Measures: Step-by-Step Process
To effectively implement compliance measures under 21 CFR 111, organizations must adhere to these essential steps:
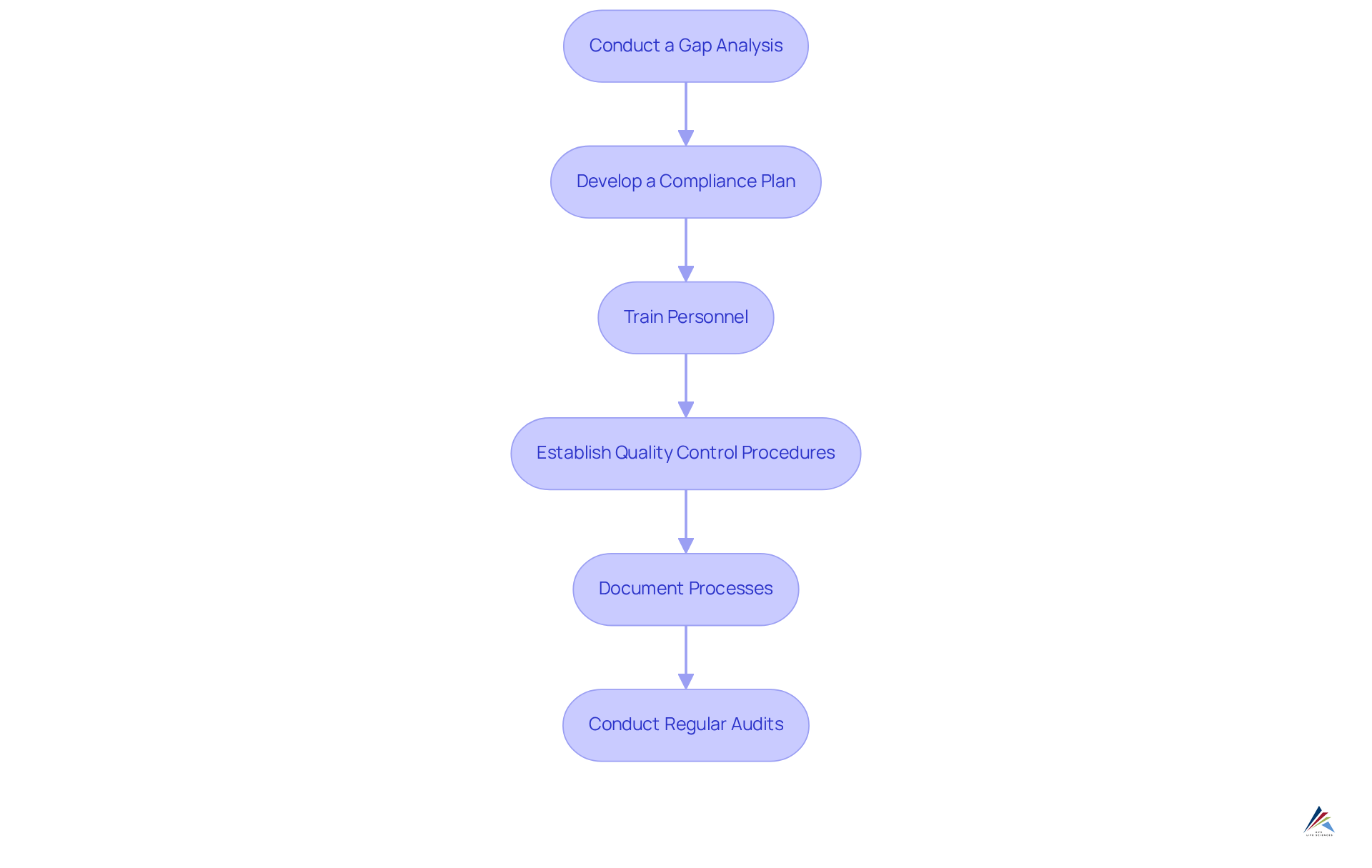
- Conduct a Gap Analysis: Evaluate current practices against the requirements of 21 CFR 111 to identify areas that need improvement. This examination should occur at least every two years, ideally alongside a , to ensure continuous adherence. As Gregory Husisian observes, performing a regulatory gap analysis is crucial for identifying areas where an organization’s adherence program may not meet regulatory standards.
- Develop a Compliance Plan: Formulate a comprehensive plan detailing how to address the identified gaps. This plan must include specific timelines and designate responsible personnel to ensure accountability. Organizations can save an average of $1.03 million through regulatory monitoring, underscoring the significance of a well-structured adherence strategy.
- Train Personnel: Provide comprehensive instruction for all staff on current Good Manufacturing Practices (cGMP) and their specific roles in maintaining compliance. Regular development sessions are essential for keeping employees informed about evolving regulations and internal policies. Employee development serves as one of the most effective cost reducers of data breaches, with breaches at developed organizations costing $260,000 less on average.
- Establish Quality Control Procedures: Implement robust procedures for testing raw materials, in-process materials, and finished products to confirm they meet established specifications. This proactive approach mitigates risks associated with non-compliance.
- Document Processes: Maintain meticulous documentation of all manufacturing processes, quality control measures, and training records. Thorough documentation is vital for demonstrating adherence during audits and inspections.
- Conduct Regular Audits: Arrange internal evaluations to assess adherence levels and identify opportunities for ongoing enhancement. Frequent audits can save entities an average of $2.86 million, highlighting the financial advantages of upholding regulations. These audits not only assist in maintaining standards but also promote a culture of accountability and transparency within the organization.
By diligently following these steps, organizations can ensure they meet the necessary regulatory standards, such as 21 CFR 111, effectively, thereby safeguarding their operations and reputation in the competitive landscape of dietary supplement manufacturing. Furthermore, leveraging technology, such as AI and automation, can significantly enhance regulatory efficiency and effectiveness.
Establish Documentation and Record-Keeping Practices
To establish effective documentation and record-keeping practices in the pharmaceutical industry, it is essential to address with a strategic approach. Consider the following steps:
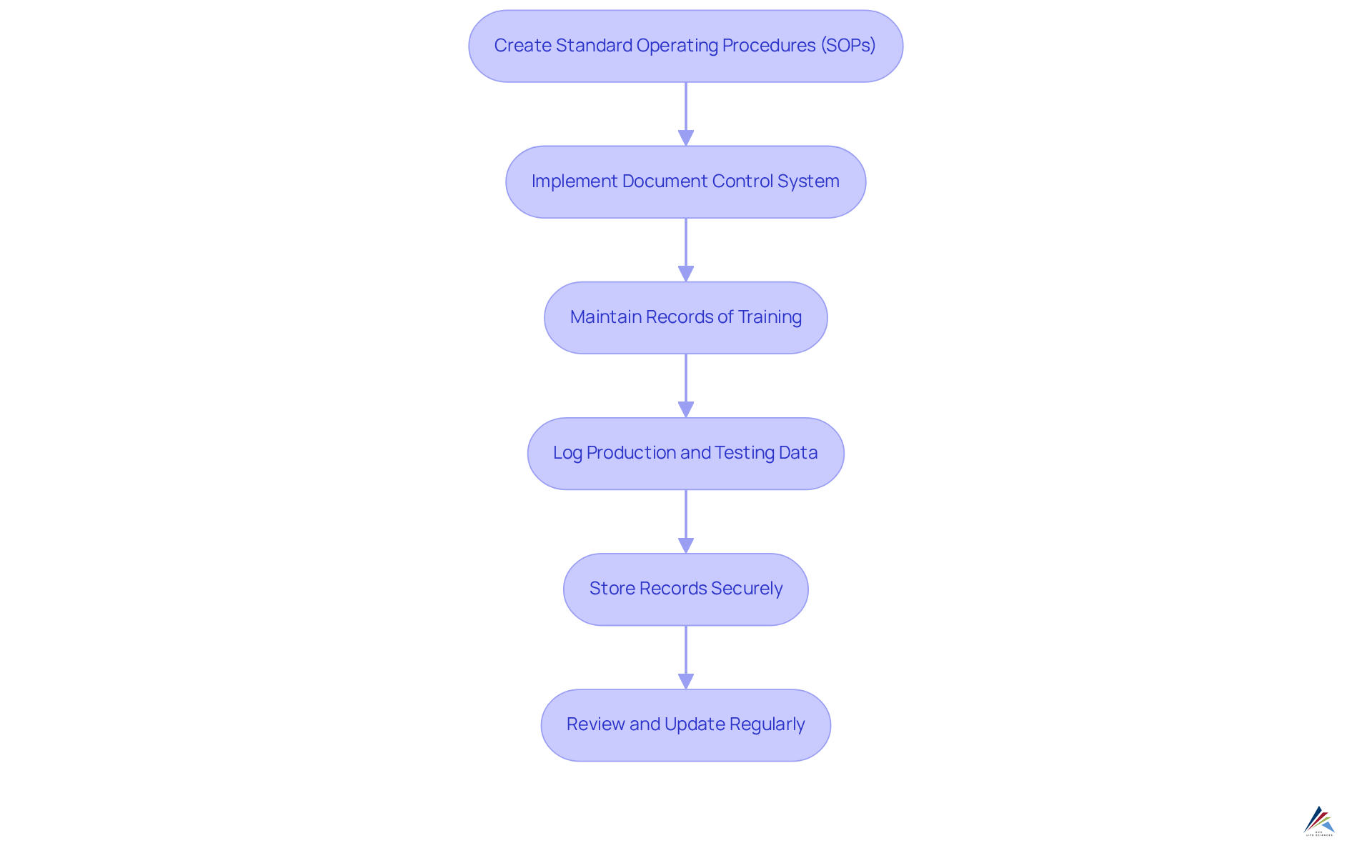
- Create Standard Operating Procedures (SOPs): Develop comprehensive SOPs for all critical processes, including manufacturing, quality control, and training. This guarantees consistency and adherence across operations.
- Implement a Document Control System: Utilize a robust document control system to manage document versions, ensuring that only the most current and approved documents are in circulation. This minimizes errors and enhances compliance. As noted, "Forty-eight percent of staff say it's hard to find documents quickly," underscoring the necessity for effective document management.
- Maintain Records of Training: Document all training sessions meticulously, including dates, attendees, and topics covered. This not only aids in adhering to regulations but also showcases a dedication to employee growth and operational excellence.
- Log Production and Testing Data: Keep detailed logs of production batches, testing results, and any deviations from established procedures. Precise record-keeping is crucial for traceability and accountability during regulatory audits.
- Store Records Securely: Ensure that all records are stored securely and are easily accessible for audits and inspections. A secure storage solution safeguards sensitive information and enables rapid retrieval during regulatory checks.
- Review and Update Regularly: Periodically review documentation practices to ensure they remain compliant with current regulations and industry standards. Frequent updates assist entities in adjusting to changing regulatory demands and enhancing operational effectiveness.
Implementing these effective documentation practices not only aids in adhering to 21 CFR 111 regulations but also improves overall operational efficiency. Given that "91 percent of data professionals report that data quality issues hurt company performance," prioritizing these practices is essential for businesses to thrive in the competitive pharmaceutical landscape.
Overcome Compliance Challenges: Strategies for Success
To effectively navigate common compliance challenges, organizations must adopt the following strategies:
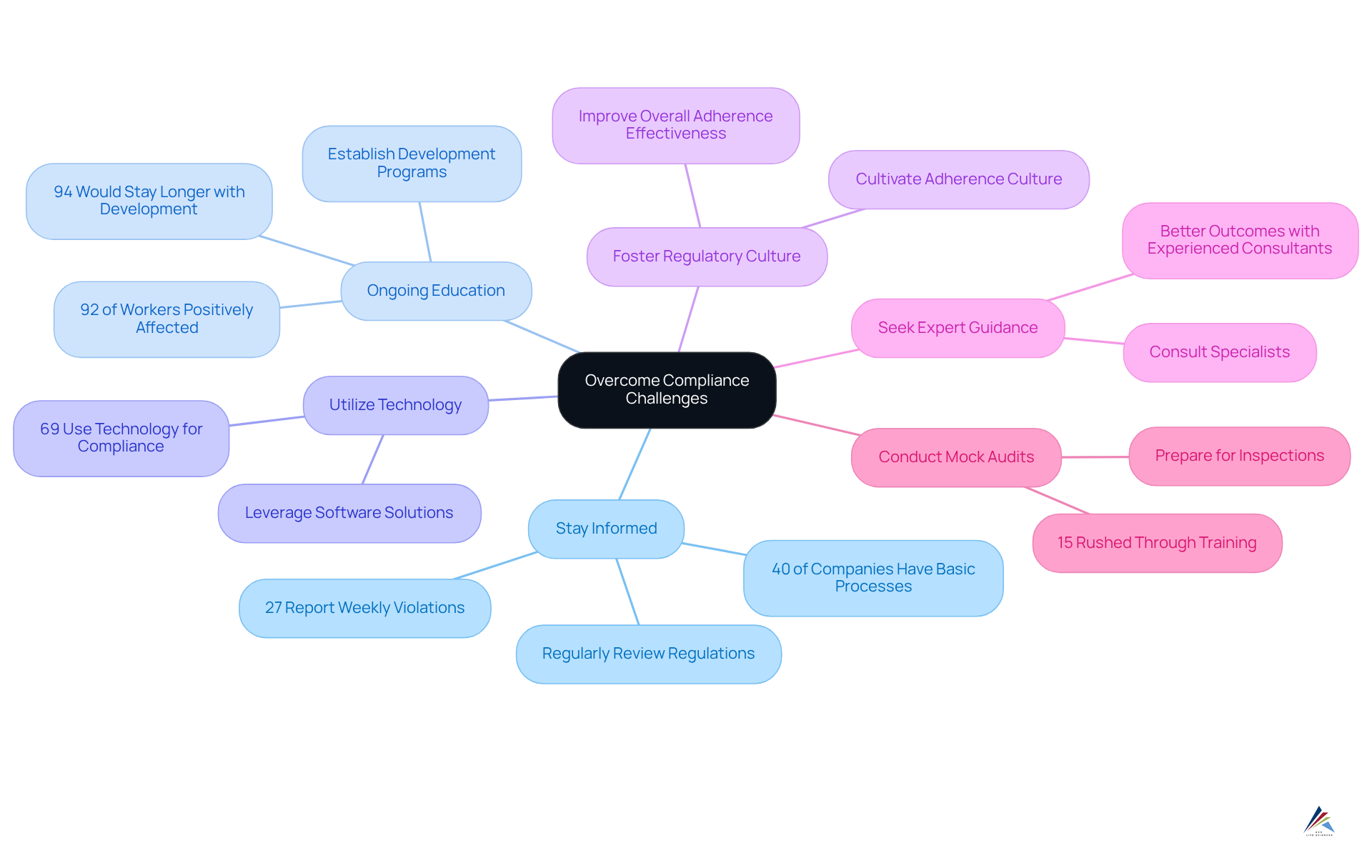
- Stay informed by regularly reviewing updates to 21 CFR 111 and other relevant regulations to ensure continuous adherence. This proactive strategy is crucial, as 40% of companies categorize their regulatory processes as basic or reactive, emphasizing the need for vigilance. Moreover, with 27% of companies indicating weekly violations or attacks, the necessity for enhancing regulatory processes cannot be overstated.
- Participate in ongoing education: Establish continual development programs for staff to keep them informed on regulatory standards and optimal methods. Data indicate that well-organized development initiatives positively influence 92% of workers, boosting engagement and awareness of regulations. Additionally, 94% of employees would remain with a firm longer if it invested in their learning and development, underscoring the importance of continuous training in employee retention.
- Utilize technology: Leverage software solutions to track adherence metrics, manage documentation, and facilitate audits. With 69% of companies to aid in regulatory processes, incorporating these tools can simplify operations and enhance efficiency.
- Foster a regulatory culture: Cultivate a culture of adherence within the organization, ensuring that all employees understand their roles in maintaining standards. This cultural shift can significantly improve overall adherence effectiveness.
- Seek expert guidance: Consult with specialists to identify potential gaps and develop tailored strategies for improvement. As Paul Koziarz emphasizes, businesses must remain vigilant and continuously challenge themselves to stay compliant. Organizations that engage with experienced consultants often report better outcomes in managing compliance-driven projects.
- Conduct mock audits: Perform internal mock audits to prepare for actual inspections and identify areas for improvement. This practice not only improves preparedness but also aids in identifying weaknesses before they escalate into problems. Notably, 15% of participants hurried through required training, emphasizing the necessity of careful preparation via mock audits.
By proactively addressing these challenges, organizations can significantly enhance their compliance posture and mitigate the risk of regulatory issues.
Conclusion
Ensuring compliance with 21 CFR 111 transcends mere regulatory obligation; it is a fundamental pillar of quality and safety in dietary supplement manufacturing. This framework delineates essential guidelines that, when executed adeptly, protect consumer health and enhance company reputation. By emphasizing quality control, personnel training, facility standards, and meticulous record-keeping, organizations can adeptly navigate the complexities of compliance while cultivating trust with their customers.
This article presents a systematic approach to achieving compliance through a series of well-defined steps:
- Conducting gap analyses
- Crafting compliance plans
- Training personnel
- Establishing quality control procedures
- Maintaining thorough documentation
These are all pivotal actions that contribute to a robust compliance strategy. Moreover, embracing technology and nurturing a culture of regulatory awareness can significantly bolster an organization’s capacity to meet and exceed compliance standards.
Ultimately, a steadfast commitment to adhering to 21 CFR 111 not only mitigates risks associated with non-compliance but also positions organizations as vanguards in the dietary supplement industry. By perceiving compliance as an ongoing journey rather than a singular task, businesses can ensure responsiveness to evolving regulations while perpetually enhancing their operational practices. Engaging with these principles and strategies will empower organizations to flourish in a competitive landscape, ultimately benefiting both their bottom line and the health of the consumers they serve.
Frequently Asked Questions
What is 21 CFR 111?
21 CFR 111 outlines the Current Good Manufacturing Practices (cGMP) for dietary supplements, establishing key principles and requirements for compliance.
Why is quality control important in dietary supplements?
Quality control is vital to ensure dietary supplements consistently meet safety and quality standards. Failure to establish product specifications can lead to significant regulatory issues, as indicated by recent FDA observations.
What are the key components of quality control according to 21 CFR 111?
Key components include establishing product specifications for identity, purity, strength, and composition to ensure the safety and quality of dietary supplements.
Why is personnel training necessary in manufacturing dietary supplements?
Comprehensive training for all employees involved in manufacturing is crucial to ensure they understand and comply with 21 CFR 111 requirements, which is essential for maintaining product integrity.
What facility requirements are specified in 21 CFR 111?
Facilities must maintain a clean and sanitary manufacturing environment to prevent contamination and safeguard product quality.
What is the importance of record keeping in the manufacturing process?
Thorough documentation of all processes, including production, testing, and distribution, is necessary for traceability and accountability, aiding in regulatory compliance and operational transparency.
What are the consequences of non-compliance with 21 CFR 111?
Non-compliance can lead to severe legal repercussions, including the FDA's ability to seize drugs deemed 'adulterated' due to cGMP violations, as well as damage to brand trust.
How does adherence to 21 CFR 111 impact the dietary supplement industry?
Effective application of cGMP practices in accordance with 21 CFR 111 is increasingly recognized as a hallmark of trustworthy dietary supplement producers, improving adherence rates and operational efficiencies.
What role do dietary supplements play in consumer health according to industry leaders?
Dietary supplements have become an essential part of many Americans’ health routines, highlighting the importance of adherence to quality standards in maintaining consumer trust.
