10 Ways Engineering Consultancy Enhances Pharmaceutical Compliance

Overview
Engineering consultancy enhances pharmaceutical compliance through specialized knowledge and tailored strategies that effectively address complex regulatory requirements, including Good Manufacturing Practices (GMP) and Quality System Regulations (QSR). This approach not only improves operational efficiency but also significantly reduces the risk of non-compliance. By leveraging these specialized strategies, organizations can navigate the intricate landscape of regulatory compliance with confidence and precision.
Introduction
The pharmaceutical industry faces relentless pressure to adhere to stringent compliance standards, a challenge that intensifies as regulations grow more complex and scrutiny escalates. Engineering consultancy emerges as a pivotal ally in this landscape, providing specialized expertise that not only assists companies in navigating the intricate web of regulations but also enhances operational efficiency and product quality.
As many organizations grapple with the evolving compliance requirements, a pressing question surfaces: how can engineering consultancy effectively empower pharmaceutical companies to not only meet but exceed these standards? By ensuring both regulatory adherence and a competitive advantage, these consultancies play a crucial role in shaping the future of the industry.
AVS Life Sciences: Comprehensive Expertise in Validation and Quality Compliance
distinguishes itself in the through its profound expertise in validation and . By prioritizing and , the company delivers customized solutions that empower clients to meet stringent compliance standards across diverse sectors, including biopharmaceuticals, medical devices, and nutraceuticals.
With a dedicated team of over 300 seasoned professionals, AVS adopts a hands-on approach to devising and executing effective . This commitment not only enhances operational success but also positions AVS as a reliable partner for organizations navigating the complexities of compliance environments.
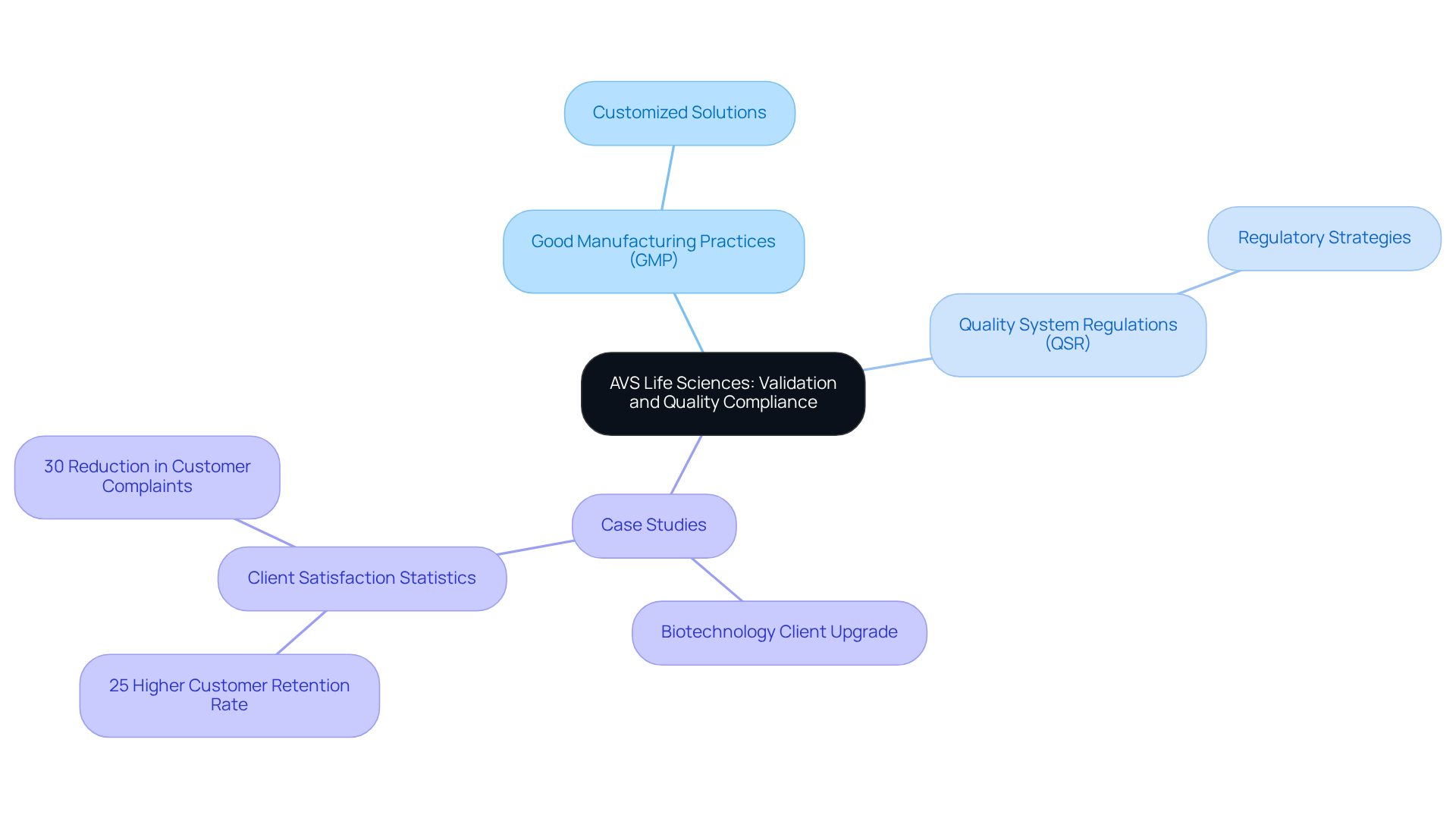
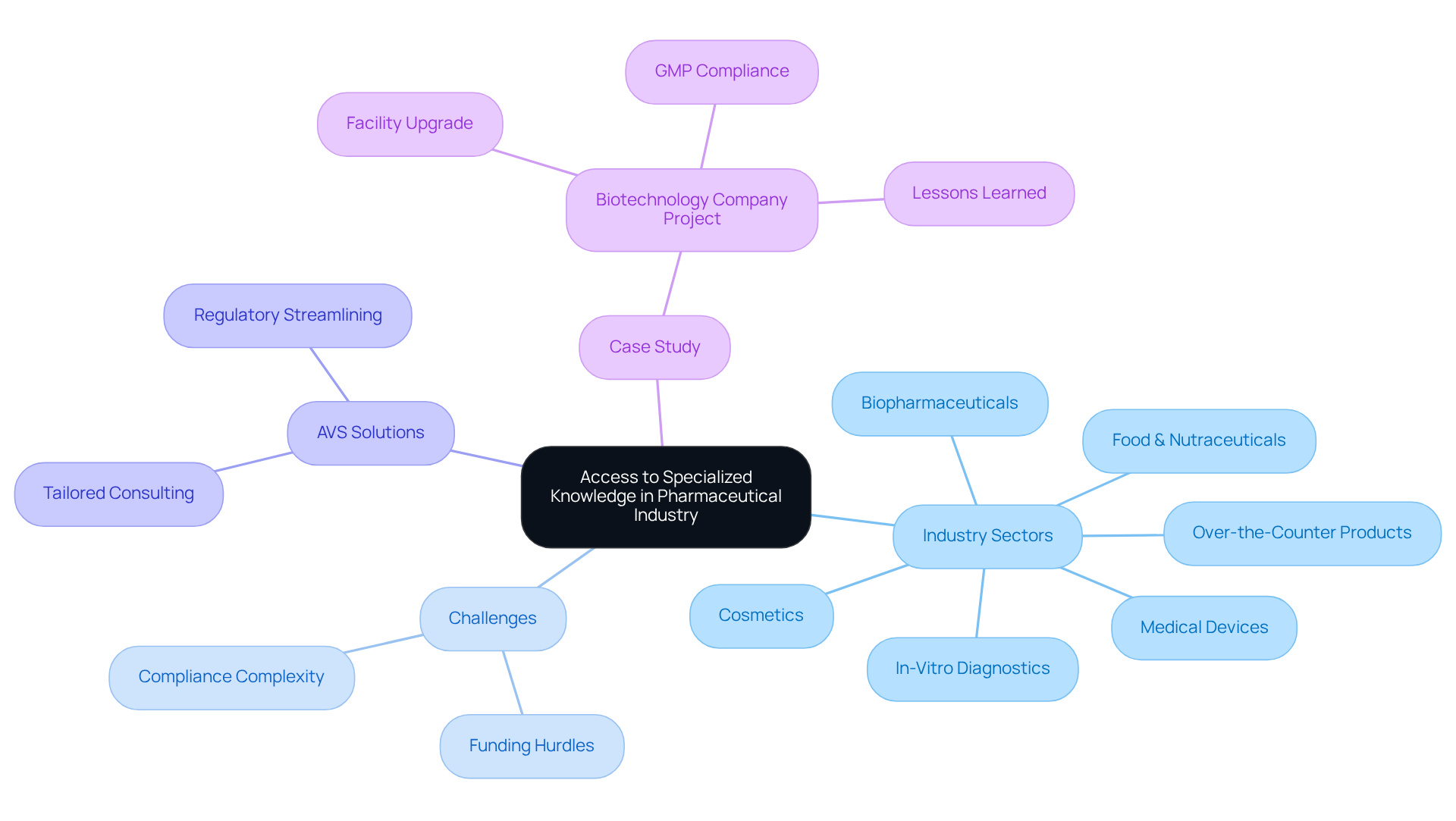
A transformative case study underscores this dedication: AVS supported a leading biotechnology client in upgrading their from a Biosafety Level 1 GMP setting to a Level 2 GMP environment. This project was completed on schedule and within budget, demonstrating AVS's capability to ensure compliance. The documentation efforts to establish full traceability were deemed satisfactory by the client’s assurance team, further reinforcing AVS's esteemed reputation within the industry.
Enhanced Regulatory Compliance: Navigating Complex Standards
An is essential for enhancing compliance with regulations, providing organizations with the specialized knowledge and resources necessary to navigate the complex landscape of standards such as , International Organization for Standardization (ISO), and Quality System Regulations (QSR). AVS Life Sciences adopts a systematic approach to ensure that all processes align meticulously with compliance standards, thereby significantly mitigating the risk of non-adherence.
At AVS Life Sciences, consultants collaborate closely with clients to devise phase-appropriate strategies that address compliance challenges head-on. This includes:
Such a tailored strategy not only streamlines the journey to market approval but also reflects current trends in , with 33% of companies actively pursuing ISO 13485 adherence.
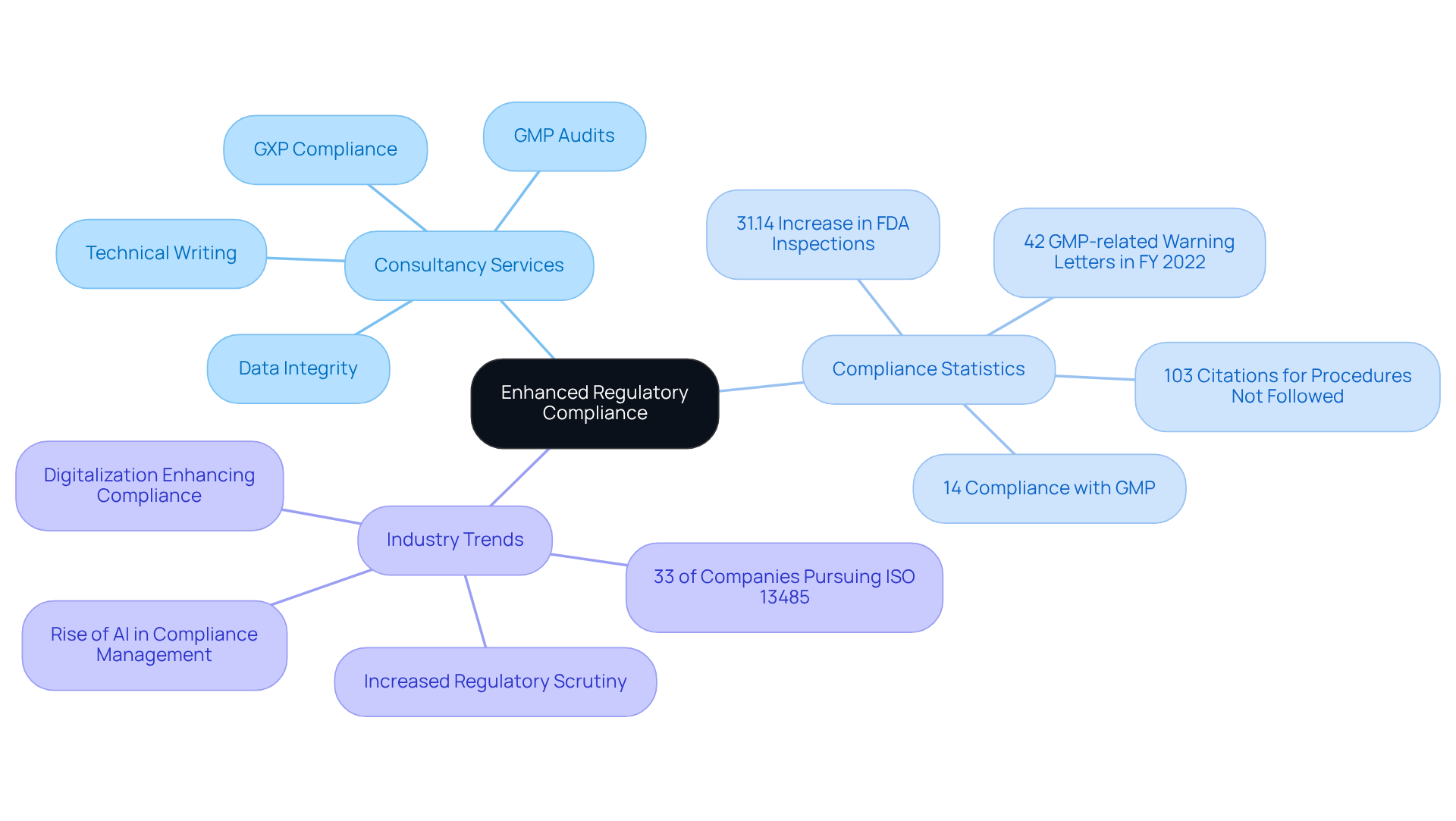
The pharmaceutical industry is currently experiencing heightened, evidenced by the FDA conducting 1,175 inspections and issuing 1,469 citations in FY 2022, marking a 31.14% increase in inspections compared to the previous year. This underscores the critical need for robust engineering consultancy to effectively manage evolving regulatory challenges.
By leveraging expert knowledge and established methodologies, ultimately enhancing their operational efficiency and market readiness. Oversight personnel are encouraged to engage with AVS Life Sciences' engineering advisory services to proactively address adherence challenges and ensure their organizations are thoroughly prepared for scrutiny.
Improved Operational Efficiency: Streamlining Processes and Reducing Costs
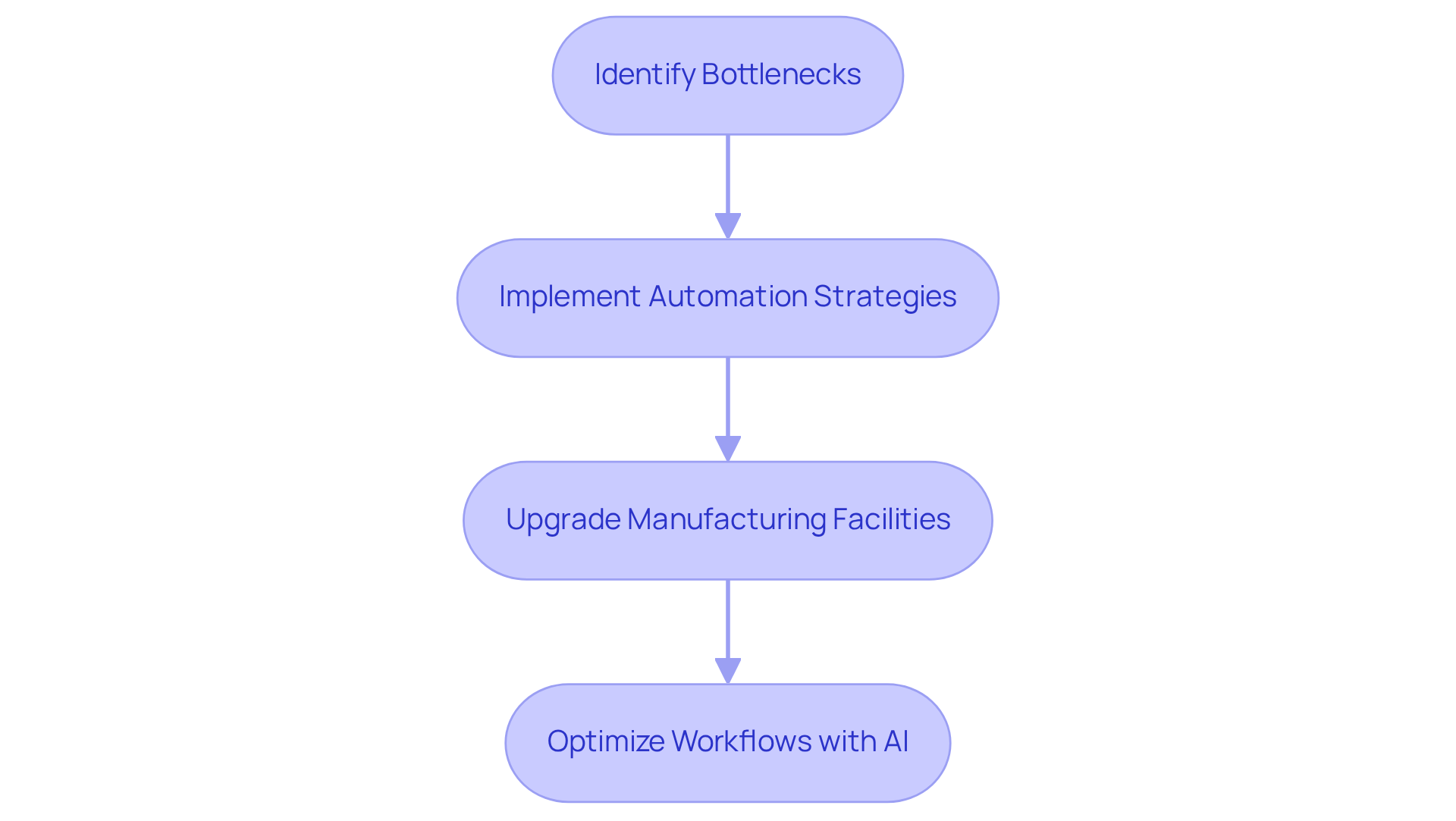
An is crucial for pharmaceutical companies in enhancing by streamlining processes. in identifying bottlenecks and inefficiencies within current workflows, employing a comprehensive range of biopharmaceutical services, including that bolster adherence and . This proactive approach allows organizations to allocate resources more effectively, leading to .
For example, AVS Life Sciences successfully aided a prominent biotechnology company in upgrading their manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility, completing the project on time and within budget. This enhancement not only ensured compliance with regulations but also improved assurance procedures, allowing the client to focus on developing medications that enhance patient well-being.
Companies utilizing AI-driven automation have reported in research and development expenses, contributing to an estimated annual savings of $26 billion for pharmaceutical firms. Furthermore, the integration of engineering consultancy has led to , as AI optimizes these workflows, reducing waste and increasing production efficiency.
Access to Specialized Knowledge: Tackling Unique Industry Challenges
The role of is pivotal in navigating the intricate landscape of pharmaceutical regulations, providing specialized knowledge essential for overcoming unique industry challenges. stands as your dedicated partner for comprehensive oversight, adherence, and quality solutions, employing a team of experts well-versed in the complexities of rule compliance. This expertise enables the delivery of tailored solutions that address specific client needs across various sectors, including biopharmaceuticals, medical devices, in-vitro diagnostics, over-the-counter products, and food & nutraceuticals.
Industry leaders emphasize the importance of continuous improvement and strategic alignment in achieving . Will Durant's assertion that "" resonates profoundly within the pharmaceutical sector, where maintaining high standards is essential. By cultivating a culture of quality and leveraging specialized knowledge, AVS Life Sciences empowers clients not only to meet regulatory requirements but also to excel in their product development initiatives.
Effective Risk Management: Identifying and Mitigating Potential Issues
Effective risk management is essential for ensuring compliance in the pharmaceutical sector, with engineering consultancy being pivotal in identifying and addressing potential issues. AVS Life Sciences adopts a , employing advanced tools and methods such as GXP verification and adherence to FDA regulations. This enables clients to pinpoint early in the product lifecycle. Early identification is critical; studies indicate that the typical cost of a pharmaceutical product recall can reach approximately 10 million dollars, underscoring the .
By implementing comprehensive , AVS Life Sciences empowers organizations to navigate complex compliance landscapes while upholding high standards. Current methodologies emphasize the creation of that include:
This systematic approach not only but also fosters a culture of preparedness, which is vital for achieving positive outcomes in risk management.
Case studies exemplify the in the life sciences sector. For example, AVS Life Sciences effectively assisted a leading biotechnology company in upgrading their manufacturing facility from a Biosafety Level 1 to a Level 2 GMP facility. This project adhered to stringent legal standards and ensured thorough documentation practices that demonstrated complete traceability, deemed satisfactory by the client’s quality assurance team. The integration of advanced equipment and processes during this transition resulted in enhanced operational efficiency and safety standards, enabling the manufacturer to compete more effectively in the market. Moreover, AVS Life Sciences' dedication to meticulous project execution has led to zero findings during audits, reinforcing its reputation as a reliable partner in the industry.
In 2025, organizations must remain vigilant in identifying regulatory risks throughout the product lifecycle. Continuous education and adaptation to evolving regulatory mandates are imperative, particularly as companies face intricate cyber threats that could jeopardize compliance efforts. By perceiving rather than a mere expense, organizations can effectively mitigate risks and bolster their overall operational resilience.
Robust Quality Assurance: Ensuring High Standards in Product Development
Strong assurance practices are essential for upholding within the life sciences sector. AVS Life Sciences employs extensive that adhere to industry regulations, including GXP and FDA standards, ensuring that all products meet rigorous criteria consistently. Their engineering consultancy collaborates closely with clients to establish and uphold assurance protocols that not only meet but also significantly enhance overall product excellence. This proactive approach is substantiated by data indicating that can lead to a reduction in product defects and an increase in compliance rates, ultimately safeguarding patient safety and ensuring the efficacy.
As the industry evolves, integrating advanced statistical methods—such as and —along with continuous improvement practices becomes imperative. This highlights the critical role of management systems in delivering safe and effective medications.
Comprehensive Training and Development: Keeping Staff Updated on Best Practices
Thorough are essential for ensuring personnel remain informed about best practices in . AVS Life Sciences offers tailored specifically for the biopharmaceutical and medical device industries, equipping employees with the necessary knowledge to effectively manage compliance demands and industry standards. This commitment to education not only strengthens adherence initiatives but also within organizations.
Industry leaders observe that organizations investing in employee training experience substantial improvements in . As organizations prepare for 2025, adopting will be crucial for maintaining and ensuring that staff are equipped to .
Expert Project Management: Ensuring Successful Project Execution
Effective is paramount for the within the pharmaceutical sector. At our seasoned project managers harness to meticulously oversee project timelines, budgets, and deliverables. This organized approach ensures that all are comprehensively addressed, leading to that aligns with client expectations.
Notably, statistics reveal that organizations employing are 28% less likely to experience budget overruns, highlighting the critical role of a disciplined framework in achieving project objectives.
Furthermore, case studies of successful project executions in the pharmaceutical sector demonstrate that a focus on not only enhances compliance adherence but also fosters trust and satisfaction among clients.
As the industry continues to evolve, the integration of advanced project management tools and methodologies will remain essential in navigating the complexities.
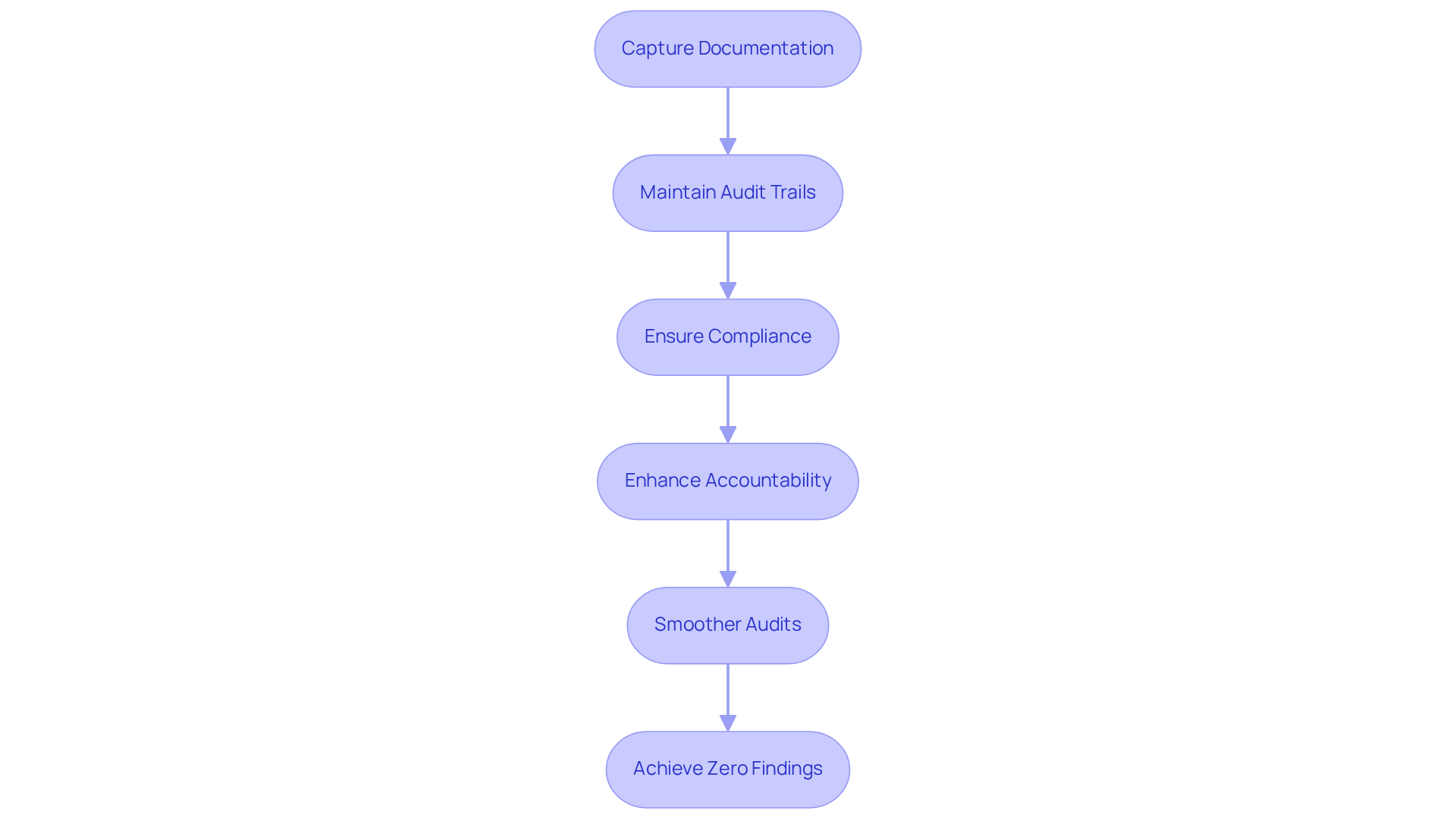
Thorough Documentation and Audit Trails: Ensuring Compliance and Transparency
In the comprehensive documentation and properly maintained are essential for ensuring adherence and transparency. AVS Life Sciences implements that meticulously capture every aspect of the . This approach not only provides clients with clear audit trails demonstrating but also significantly enhances accountability within organizations. By embracing these robust practices, AVS Life Sciences facilitates smoother audits and aids clients in navigating the complexities of compliance.
The emphasis on establishing thorough audit trails aligns with industry standards, ensuring that all documentation is accurate, clear, and recorded in real-time—crucial for upholding and other regulatory frameworks. Furthermore, the company’s unwavering commitment to is evidenced by its achievement of zero findings during audits, reinforcing its reputation with AVS Life Sciences, clients can confidently engage in that not only meet but exceed industry expectations.
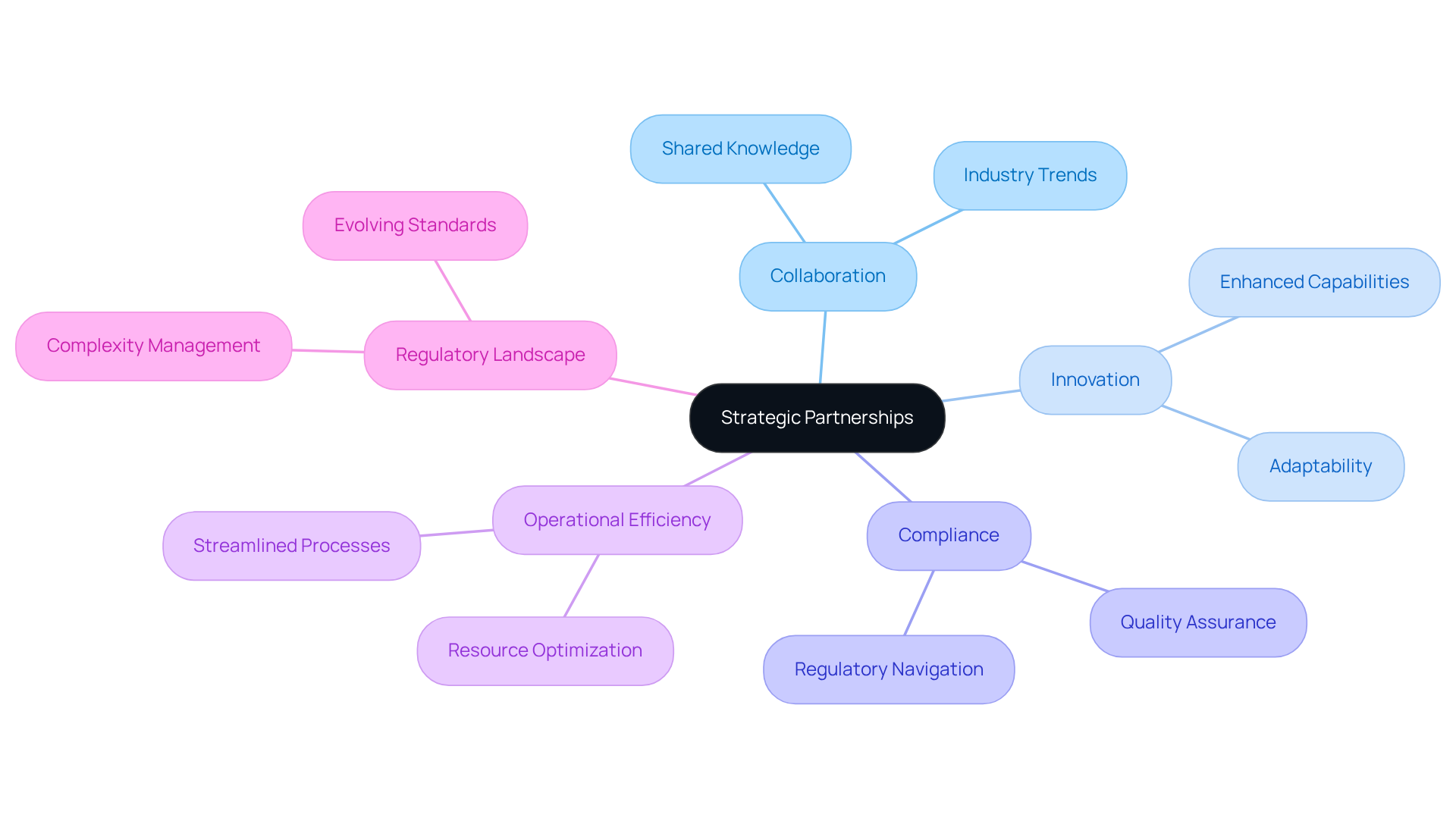
Strategic Partnerships: Enhancing Collaboration and Innovation
Are crucial in driving collaboration and innovation within the pharmaceutical sector. is committed to forging alliances with industry leaders, thereby creating an ecosystem rich in shared knowledge and resources. These partnerships not only position AVS at the forefront of but also empower clients to leverage the and quality assurance.
By integrating diverse expertise and insights, AVS amplifies its capacity to navigate complex, ultimately fostering across the life sciences spectrum. This not only enhances operational efficiency but also ensures that are both effective and adaptable.
Conclusion
AVS Life Sciences exemplifies the critical role that engineering consultancy plays in enhancing pharmaceutical compliance. By offering tailored solutions that address the complexities of regulatory standards, companies like AVS empower clients to navigate the intricate landscape of compliance effectively. This approach not only ensures adherence to regulations but also optimizes operational efficiency and reduces costs, ultimately leading to improved product development and market readiness.
Key insights throughout the article highlight the multifaceted benefits of engineering consultancy in the pharmaceutical sector. From robust quality assurance practices and comprehensive training programs to effective risk management and strategic partnerships, AVS Life Sciences showcases how specialized knowledge and methodologies contribute to sustained compliance and operational excellence. The case studies presented further illustrate the tangible impact of AVS's expertise, demonstrating significant improvements in client outcomes.
As the pharmaceutical industry continues to evolve, the importance of engaging with experienced engineering consultancies cannot be overstated. Organizations are encouraged to view compliance not merely as a regulatory obligation but as a strategic investment that fosters innovation and resilience. By prioritizing collaboration and leveraging expert knowledge, businesses can better prepare for the challenges ahead, ensuring they remain competitive and compliant in an increasingly complex environment.
Frequently Asked Questions
What expertise does AVS Life Sciences offer in the pharmaceutical industry?
AVS Life Sciences specializes in validation and quality assurance, focusing on Good Manufacturing Practices (GMP) and Quality System Regulations (QSR) to provide customized solutions for compliance in sectors such as biopharmaceuticals, medical devices, and nutraceuticals.
How does AVS Life Sciences ensure compliance for its clients?
AVS Life Sciences employs a hands-on approach with a team of over 300 professionals to devise and execute effective regulatory strategies, ensuring that clients meet compliance standards and navigate complex regulatory environments.
Can you provide an example of AVS Life Sciences' successful project?
AVS Life Sciences supported a biotechnology client in upgrading their manufacturing facility from a Biosafety Level 1 GMP setting to a Level 2 GMP environment, completing the project on schedule and within budget while ensuring compliance and satisfactory documentation.
What impact does strong management have on customer retention in the pharmaceutical industry?
According to the Aberdeen Group, organizations with robust management systems experience a 25% higher customer retention rate, highlighting the importance of quality management practices in maintaining a competitive edge.
How does AVS Life Sciences help clients with regulatory compliance challenges?
AVS Life Sciences collaborates with clients to develop phase-appropriate strategies that include comprehensive GXP compliance services, GMP audits, ensuring data integrity, and effective technical writing, which streamline the path to market approval.
What is the current state of regulatory scrutiny in the pharmaceutical industry?
The industry is facing increased regulatory scrutiny, evidenced by the FDA conducting 1,175 inspections and issuing 1,469 citations in FY 2022, marking a significant rise in inspections compared to the previous year.
How does AVS Life Sciences enhance operational efficiency for pharmaceutical companies?
AVS Life Sciences identifies bottlenecks and inefficiencies within workflows and employs biopharmaceutical services, including automation and validation strategies, to streamline processes and reduce operational costs.
What cost savings have companies experienced by utilizing AI-driven automation?
Companies using AI-driven automation have reported up to 40% reductions in research and development expenses, contributing to an estimated annual savings of $26 billion for pharmaceutical firms.
What role does AVS Life Sciences play in helping organizations prepare for regulatory scrutiny?
AVS Life Sciences provides engineering advisory services that empower organizations to proactively address adherence challenges and ensure they are well-prepared for regulatory scrutiny.
