10 Key Strategies for Pharmaceutical Regulatory Compliance

Overview
The article titled "10 Key Strategies for Pharmaceutical Regulatory Compliance" addresses the critical challenges organizations face in adhering to regulatory standards within the pharmaceutical sector. It delineates essential strategies, including:
- The implementation of a robust Quality Management System (QMS)
- The adoption of risk-based compliance approaches
- The leveraging of technology to enhance operational efficiency
These strategies are designed not merely to fulfill regulatory obligations but to transform compliance into a strategic advantage that drives growth and excellence. By embracing these methodologies, organizations can navigate the complexities of regulatory landscapes, ensuring that compliance becomes a cornerstone of their operational framework.
Introduction
In the complex world of pharmaceuticals, regulatory compliance is not merely a checkbox; it is a critical component that can dictate the success or failure of an organization. As companies navigate an increasingly intricate landscape filled with evolving regulations and stringent standards, the need for effective compliance strategies has never been more pressing. This article explores ten key strategies that empower pharmaceutical companies to not only meet regulatory requirements but also leverage compliance as a competitive advantage.
How can organizations transform their compliance efforts from a burdensome obligation into a strategic resource that enhances operational efficiency and fosters trust with stakeholders?
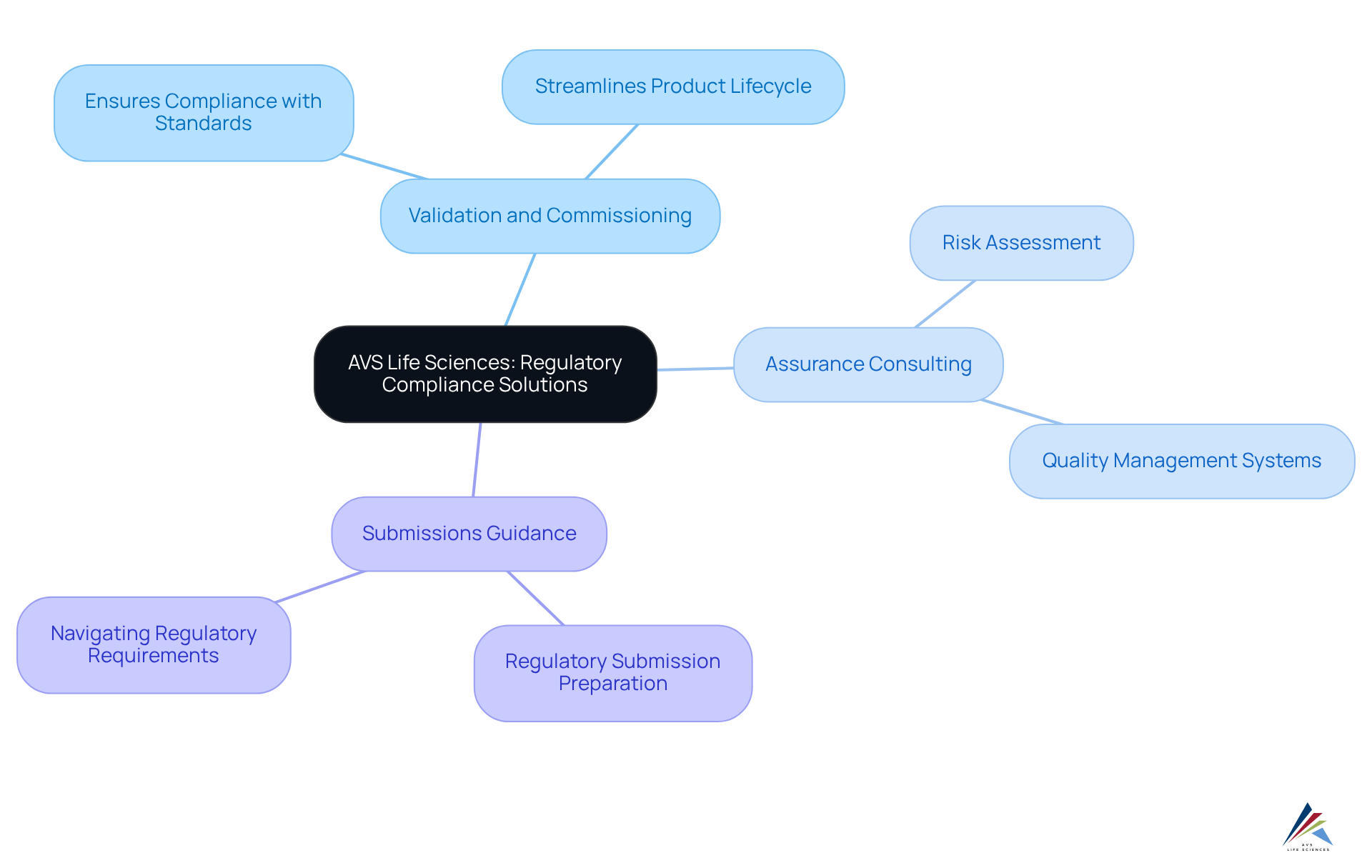
AVS Life Sciences: Comprehensive Regulatory Compliance Solutions
AVS Life Sciences offers a comprehensive suite of adherence solutions tailored for the pharmaceutical, biotechnology, and medical device sectors. Their services include:
- Validation and commissioning
- Assurance consulting
- Submissions guidance
All delivered with a hands-on approach. By focusing on empowering clients throughout the product lifecycle, AVS Life Sciences enables organizations to effectively navigate complex regulatory landscapes, ensuring pharmaceutical regulatory compliance with GxP, ISO, and QSR standards. With a proven track record and a commitment to excellence, they stand as a trusted partner in achieving regulatory success. Consider the impact of partnering with AVS Life Sciences to enhance your organization's and secure its future.
Integrated Quality Management: Ensuring Compliance and Excellence
A unified management system (QMS) is essential for ensuring pharmaceutical regulatory compliance and achieving operational superiority in the pharmaceutical sector. By aligning excellence processes with pharmaceutical regulatory compliance, firms can significantly decrease risks and enhance product excellence.
Implementing a robust QMS entails:
- Conducting regular audits
- Performing thorough documentation reviews
- Engaging in continuous improvement initiatives, including adherence to Standard Operating Procedures (SOPs) and effective internal and external auditing techniques
This proactive method not only meets pharmaceutical regulatory compliance standards but also fosters a culture of excellence within the organization. As W. Edwards Deming stated, "Enhance standards, you automatically boost productivity," highlighting the intrinsic link between standard management and operational efficiency.
Organizations that have successfully adopted these practices, such as those utilizing the Kaizen approach, often report enhanced operational excellence, demonstrating the tangible benefits of a well-structured QMS. A transformative instance of this is AVS Life Sciences' successful enhancement of a biotechnology GMP facility, where careful focus on assurance and adherence to regulations resulted in better operational outcomes.
Moreover, disregarding standards can have substantial financial consequences, as Philip Crosby pointed out, "The expense of poor standards is always greater than the expense of prevention." Thus, adopting this framework is crucial for managing the intricacies of pharmaceutical regulatory compliance while promoting a dedication to excellence throughout all tiers of the entity.
To start , entities should begin by evaluating their existing processes and pinpointing areas for enhancement, ensuring that quality becomes a shared responsibility.

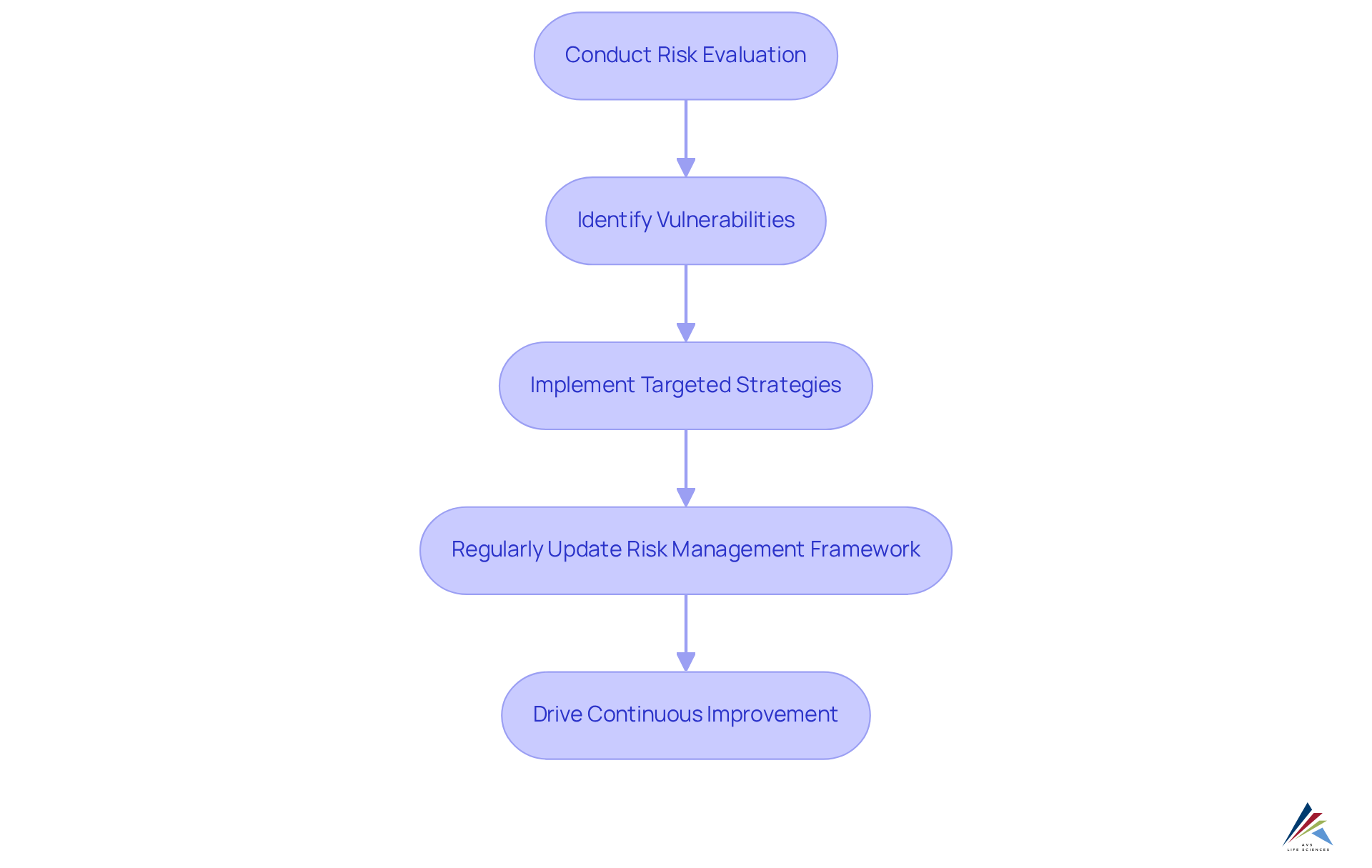
Risk-Based Compliance Approaches: Prioritizing Resources for Success
Adopting a risk-based regulatory approach empowers organizations to enhance pharmaceutical regulatory compliance by prioritizing resources effectively, focusing on areas with the highest potential impact, particularly concerning GXP and FDA regulations. By conducting comprehensive risk evaluations, businesses can identify vulnerabilities related to data integrity and implement targeted strategies to mitigate risks, thereby ensuring pharmaceutical regulatory compliance as well as adherence to Good Manufacturing Practices (GMP) and other industry standards. This approach not only enhances adherence but also significantly improves overall operational efficiency.
Organizations must regularly evaluate and update their to adapt to evolving compliance landscapes and ensure pharmaceutical regulatory compliance against emerging threats. By leveraging thorough management practices and Standard Operating Procedures (SOPs), they can maintain high operational standards and drive continuous improvement in their efforts towards pharmaceutical regulatory compliance.
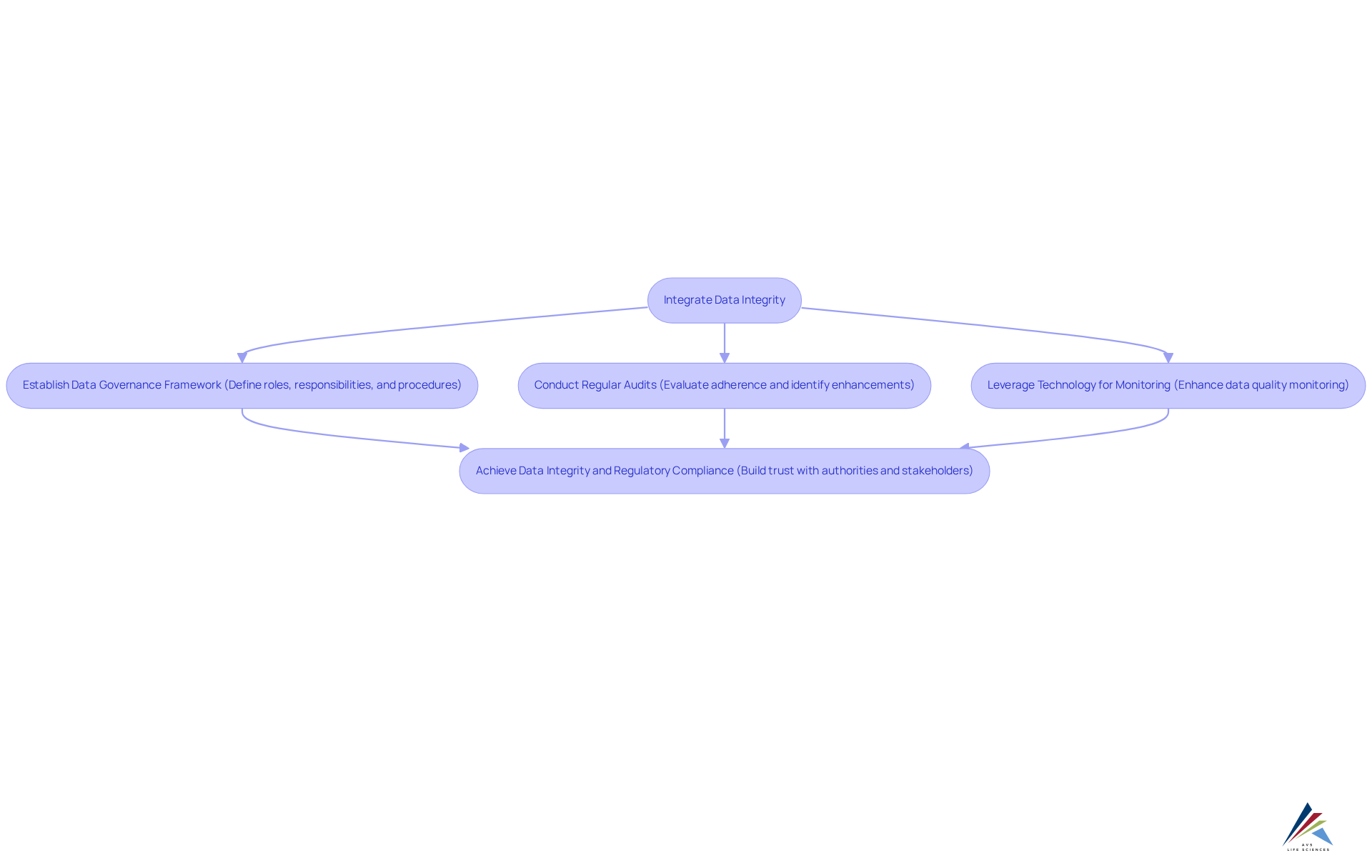
Data Integrity by Design: Building Trust and Compliance
Data integrity is paramount for ensuring pharmaceutical regulatory compliance within the pharmaceutical sector. Organizations must integrate data integrity into their processes from the very beginning, ensuring that data remains accurate, complete, and reliable throughout its lifecycle. This necessitates the establishment of robust that clearly define roles, responsibilities, and procedures for effective data management.
Regular audits are essential to evaluate adherence and pinpoint areas for enhancement, while leveraging technology can significantly improve the monitoring of data quality. By prioritizing data integrity, companies not only ensure pharmaceutical regulatory compliance but also foster trust with authorities and stakeholders, thereby bolstering their reputation within the industry.
As Peter Sondergaard aptly noted, 'Data is the new oil,' underscoring that its true value is unlocked only when it is properly governed and analyzed. Implementing these best practices empowers firms to adeptly navigate the complexities of pharmaceutical regulatory compliance.
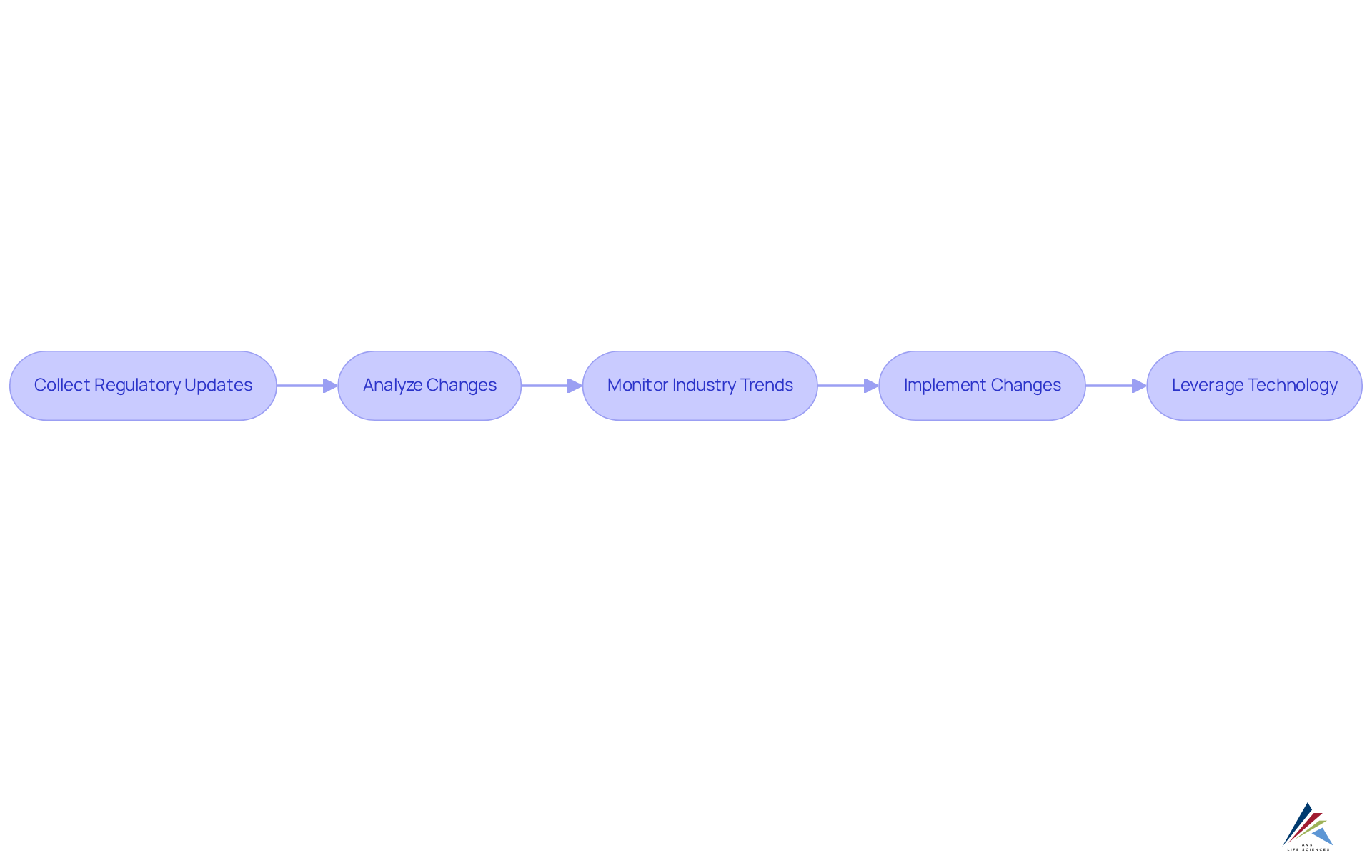
Continuous Regulatory Intelligence: Staying Ahead of Changes
Ongoing pharmaceutical regulatory compliance intelligence is essential for organizations aiming to effectively navigate the evolving landscape of pharmaceutical regulations. This involves systematically collecting and analyzing updates from oversight bodies, such as GXP and FDA regulations, to stay ahead of impending changes. Establishing robust processes for monitoring pertinent regulations, industry trends, and guidance is vital. For example, AVS Life Sciences successfully supported a leading biotechnology company in upgrading their GMP facility, ensuring that quality management practices, including standard operating procedures (SOPs), were meticulously implemented throughout the transition.
Leveraging technology and data analysis significantly enhances compliance intelligence capabilities. By utilizing advanced tools, companies can monitor changes in real-time, allowing for swift responses to compliance shifts. This proactive strategy mitigates the with pharmaceutical regulatory compliance and fosters a culture of agility within the organization. Statistics reveal that organizations that effectively track legal changes experience a marked improvement in adherence rates, underscoring the necessity of continuous legal awareness in maintaining high standards of quality and safety within the pharmaceutical industry.
Transform Compliance into a Competitive Edge: Strategic Approaches
Organizations can elevate adherence from a regulatory necessity to a strategic advantage by embedding it into their core business strategies. This transformation necessitates cultivating a culture of that permeates all levels of the organization and utilizing regulatory data to inform decision-making processes.
By demonstrating a strong dedication to regulations, companies not only enhance their reputation but also build trust with stakeholders, paving the way for growth opportunities. Companies that acknowledge adherence as a strategic resource are more skilled at managing legal complexities and capturing market opportunities.
For instance, leading organizations are increasingly incorporating pharmaceutical regulatory compliance into their strategic frameworks to ensure alignment with their overall business objectives. This proactive approach not only mitigates risks but also positions them favorably in a competitive landscape, particularly as the industry anticipates significant regulatory changes in 2025.
Based on a recent survey, 75% of worldwide life sciences leaders are hopeful about 2025, emphasizing the favorable perspective on regulatory adherence as a strategic advantage. Moreover, with a third of executives indicating worry about possible alterations to US regulations, the importance of incorporating pharmaceutical regulatory compliance into business strategies cannot be overstated. Companies that fail to do so risk not only regulatory penalties but also reputational damage and lost market opportunities.
Technology-Enabled Compliance: Streamlining Processes for Efficiency
Technology is indispensable for optimizing processes related to pharmaceutical regulatory compliance and enhancing operational efficiency. Organizations must invest in management systems that:
- Automate repetitive tasks
- Streamline data collection
- Deliver real-time insights into pharmaceutical regulatory compliance
By leveraging advanced technologies such as artificial intelligence and machine learning, companies can significantly enhance their monitoring capabilities for pharmaceutical regulatory compliance, swiftly identify potential regulatory issues, and enact proactive measures. This integration not only alleviates manual workloads but also improves the of pharmaceutical regulatory compliance data.
Notably, organizations utilizing AI-powered solutions have experienced:
- A marked reduction in decision-making times
- An increase in stakeholder satisfaction
This underscores the transformative potential of these technologies in enhancing pharmaceutical regulatory compliance.

Training Excellence: Building Compliance Capability
Developing adherence capability necessitates a strong emphasis on training quality, particularly in relation to computer system validation (CSV) processes. Organizations must establish comprehensive training programs that educate employees about pharmaceutical regulatory compliance, including:
- Regulatory obligations
- Adherence procedures
- Best practices
- The phases of CSV as outlined in the Good Automated Manufacturing Practices (GAMP) 5 Guide
This includes a thorough understanding of the V-Model, which emphasizes assessments at each validation stage. Regular training sessions, workshops, and e-learning modules are essential for reinforcing knowledge of regulations and ensuring that employees understand critical steps such as:
- User Requirement Specifications (URS)
- Installation Qualification (IQ)
- Operational Qualification (OQ)
- Performance Qualification (PQ) testing
All in the context of pharmaceutical regulatory compliance and the importance of meticulous documentation during the reporting phase. By fostering a culture of continuous education around these essential processes, organizations can enhance their pharmaceutical regulatory compliance and reduce the risk of non-conformity, thereby positioning themselves as leaders in quality management and oversight within the life sciences sector.
Navigating International Regulatory Harmonization: Ensuring Global Compliance
Navigating the challenges of pharmaceutical regulatory compliance and international harmonization presents significant difficulties for organizations in the global pharmaceutical marketplace. To thrive, businesses must remain vigilant regarding and adapt their adherence strategies accordingly across diverse jurisdictions. Engaging with governing entities and participating in industry forums are essential steps in this complex process. Leveraging local knowledge can further empower organizations to navigate the intricacies of global regulations.
By aligning their practices with internationally accepted standards, such as the ICH guidelines and the electronic Common Technical Document (eCTD), companies can enhance their market access while ensuring pharmaceutical regulatory compliance and minimizing the risk of non-compliance. Notably, harmonization efforts have proven effective in accelerating international drug approvals and enhancing pharmaceutical regulatory compliance, as evidenced by the FDA and EMA's acceptance of similarly structured submissions.
Furthermore, the integration of technology—such as Regulatory Information Management Systems (RIMS) and artificial intelligence—is revolutionizing regulatory strategies and improving pharmaceutical regulatory compliance by facilitating real-time monitoring and streamlining submissions.
AVS Life Sciences exemplifies the importance of standards adherence through their comprehensive GXP oversight services, which include numerous GMP assessments for APIs, drug products, and testing facilities. Their recent case study highlights a successful enhancement of a biotechnology GMP facility, where AVS Life Sciences ensured compliance with standards and regulations during the transition process. This collaboration not only enabled the client to produce medication using lentivirus vector material but also resulted in significant improvements in their control processes.
As the regulatory landscape evolves, companies that proactively refine their strategies for pharmaceutical regulatory compliance—like those supported by AVS Life Sciences—will effectively navigate legal challenges and position themselves for success in a competitive global market.

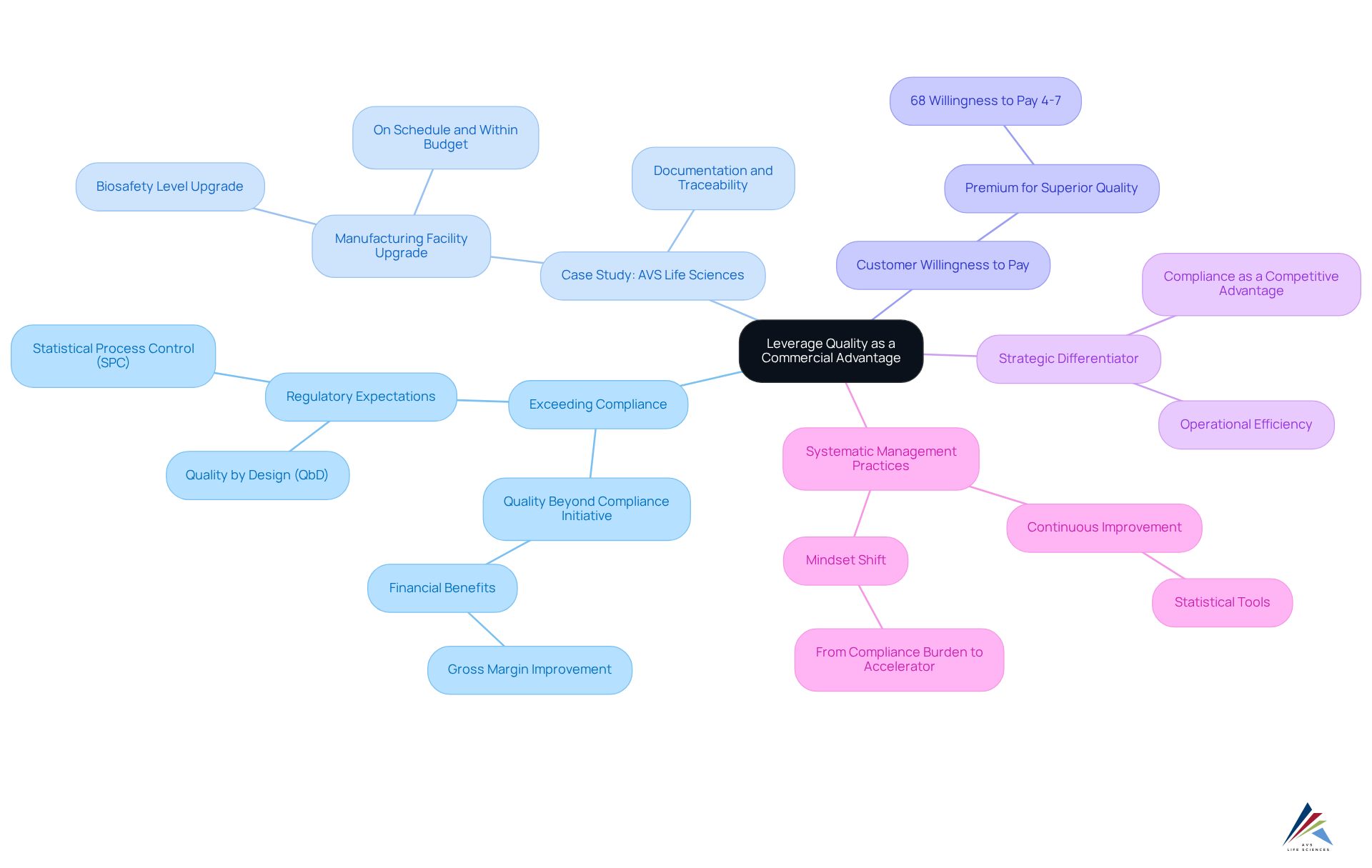
Leverage Quality as a Commercial Advantage: Beyond Compliance
Entities in the pharmaceutical industry can transform management standards into significant business advantages by not only meeting but exceeding regulatory expectations. This proactive strategy results in the delivery of superior products and services, ultimately enhancing customer satisfaction and fostering brand loyalty. For instance, firms that implement a 'Quality Beyond Compliance' initiative have reported improvements in gross margins exceeding 12%, demonstrating the financial benefits of prioritizing excellence, which aligns with AVS Life Sciences' commitment to management standards and regulatory adherence.
A prime example of this is AVS Life Sciences' collaboration with a prominent biotechnology company in San Francisco, where they successfully upgraded the client's manufacturing facility from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. This project was completed on schedule and within budget, exemplifying how AVS Life Sciences enhances control and compliance in the biotech sector. The meticulous documentation efforts ensured complete traceability, as confirmed by the client's assurance team, further underscoring the importance of robust management practices.
Moreover, a commitment to excellence can significantly shape customer perceptions. Research reveals that 68% of healthcare purchasers are willing to pay a premium of 4-7% for products that demonstrate superior quality performance. This willingness highlights the competitive advantage derived from surpassing regulatory standards.
Successful organizations view compliance as a strategic differentiator rather than merely a regulatory obligation. For example, the aforementioned biotechnology producer was able to focus on developing medicines to improve patient well-being, thanks to AVS Life Sciences' support during the transition. This shift not only enhanced operational efficiency but also bolstered their market reputation, illustrating how AVS Life Sciences can help organizations achieve similar outcomes. Importantly, throughout the project, insights gained regarding the barcode scanner issue emphasized the necessity of comprehensive testing and validation procedures, ultimately leading to improved results.
Integrating quality management into business strategies not only ensures adherence but also promotes long-term growth. As one industry leader remarked, "We ceased regarding adherence as something that hinders us and began to see it as something that accelerates us." This mindset shift is essential for navigating the complexities of the pharmaceutical landscape, where regulatory expectations are continually evolving.
Furthermore, adopting systematic management practices, such as Statistical Process Control (SPC), can facilitate ongoing improvement and assist entities in surpassing regulatory expectations. Ultimately, organizations that are better positioned to thrive in a competitive marketplace, ensuring they not only meet but exceed compliance requirements, thereby enhancing customer satisfaction and driving business success.
Conclusion
AVS Life Sciences asserts that effective pharmaceutical regulatory compliance transcends mere obligation; it serves as a strategic advantage that can significantly elevate operational excellence and market competitiveness. By integrating comprehensive compliance strategies, organizations can adeptly navigate the complexities of regulatory landscapes, ensuring adherence to GxP, ISO, and QSR standards while cultivating a culture of quality and continuous improvement.
The article delineates several pivotal strategies for achieving pharmaceutical regulatory compliance. These include:
- The implementation of robust Quality Management Systems (QMS)
- The adoption of risk-based compliance approaches
- The prioritization of data integrity
- The leveraging of technology for enhanced efficiency
Each strategy is instrumental in fortifying compliance efforts, mitigating risks, and augmenting overall operational efficiency. Furthermore, the significance of ongoing regulatory intelligence and training excellence is paramount, as these elements empower organizations to adapt to evolving compliance requirements and enable employees to uphold high standards.
Ultimately, the journey toward regulatory compliance should be perceived as a pathway to excellence rather than a mere obligation. Organizations that embrace compliance as a core value are strategically positioned to thrive within a competitive landscape, ensuring not only adherence to regulations but also the delivery of superior products and services. By adopting these strategies, companies will not only mitigate risks but also enhance customer satisfaction, drive business success, and secure their future in the dynamic pharmaceutical industry.
Frequently Asked Questions
What services does AVS Life Sciences offer for regulatory compliance?
AVS Life Sciences offers a comprehensive suite of adherence solutions including validation and commissioning, assurance consulting, and submissions guidance tailored for the pharmaceutical, biotechnology, and medical device sectors.
How does AVS Life Sciences support clients in regulatory compliance?
AVS Life Sciences empowers clients throughout the product lifecycle, helping them navigate complex regulatory landscapes to ensure compliance with GxP, ISO, and QSR standards.
What is the importance of a unified Quality Management System (QMS) in the pharmaceutical sector?
A unified QMS is essential for ensuring pharmaceutical regulatory compliance and achieving operational superiority by aligning excellence processes with compliance standards, which helps decrease risks and enhance product quality.
What are the key components of implementing a robust QMS?
Key components include conducting regular audits, performing thorough documentation reviews, and engaging in continuous improvement initiatives, such as adherence to Standard Operating Procedures (SOPs) and effective auditing techniques.
What are the benefits of adopting a risk-based compliance approach?
A risk-based compliance approach allows organizations to prioritize resources effectively, focusing on areas with the highest potential impact, which enhances compliance with GXP and FDA regulations while improving overall operational efficiency.
How can organizations ensure their risk management frameworks remain effective?
Organizations must regularly evaluate and update their risk management frameworks to adapt to evolving compliance landscapes and emerging threats, ensuring ongoing pharmaceutical regulatory compliance.
What is the significance of adhering to standards in the pharmaceutical industry?
Adhering to standards is crucial as it not only meets regulatory compliance but also fosters a culture of excellence within the organization, ultimately leading to enhanced productivity and operational efficiency.
