10 Key Strategies for Continued Process Verification (CPV) Success

Overview
The article emphasizes key strategies for achieving success in continued process verification (CPV) within the pharmaceutical sector. It highlights the critical need for:
- Robust data collection
- Statistical analysis
- Cross-functional collaboration
as essential components to ensure compliance with regulatory standards and enhance product quality throughout the manufacturing lifecycle. By integrating these strategies, organizations can effectively address compliance challenges, paving the way for improved operational efficiency and product integrity. The commitment to these practices not only fosters regulatory adherence but also cultivates a culture of continuous improvement, ultimately driving better outcomes in product quality. Engaging with AVS Life Sciences can provide the necessary tools and insights to implement these strategies effectively.
Introduction
The pharmaceutical industry is navigating an increasingly complex landscape of regulatory requirements and quality demands, making continued process verification (CPV) more crucial than ever. This article explores ten key strategies designed to empower organizations in enhancing their CPV efforts, ensuring compliance and elevating product quality throughout the manufacturing lifecycle. As companies pursue excellence, they frequently encounter the challenge of integrating innovative solutions while upholding rigorous standards. What are the most effective approaches to successfully navigate this intricate terrain and achieve sustained success in CPV?
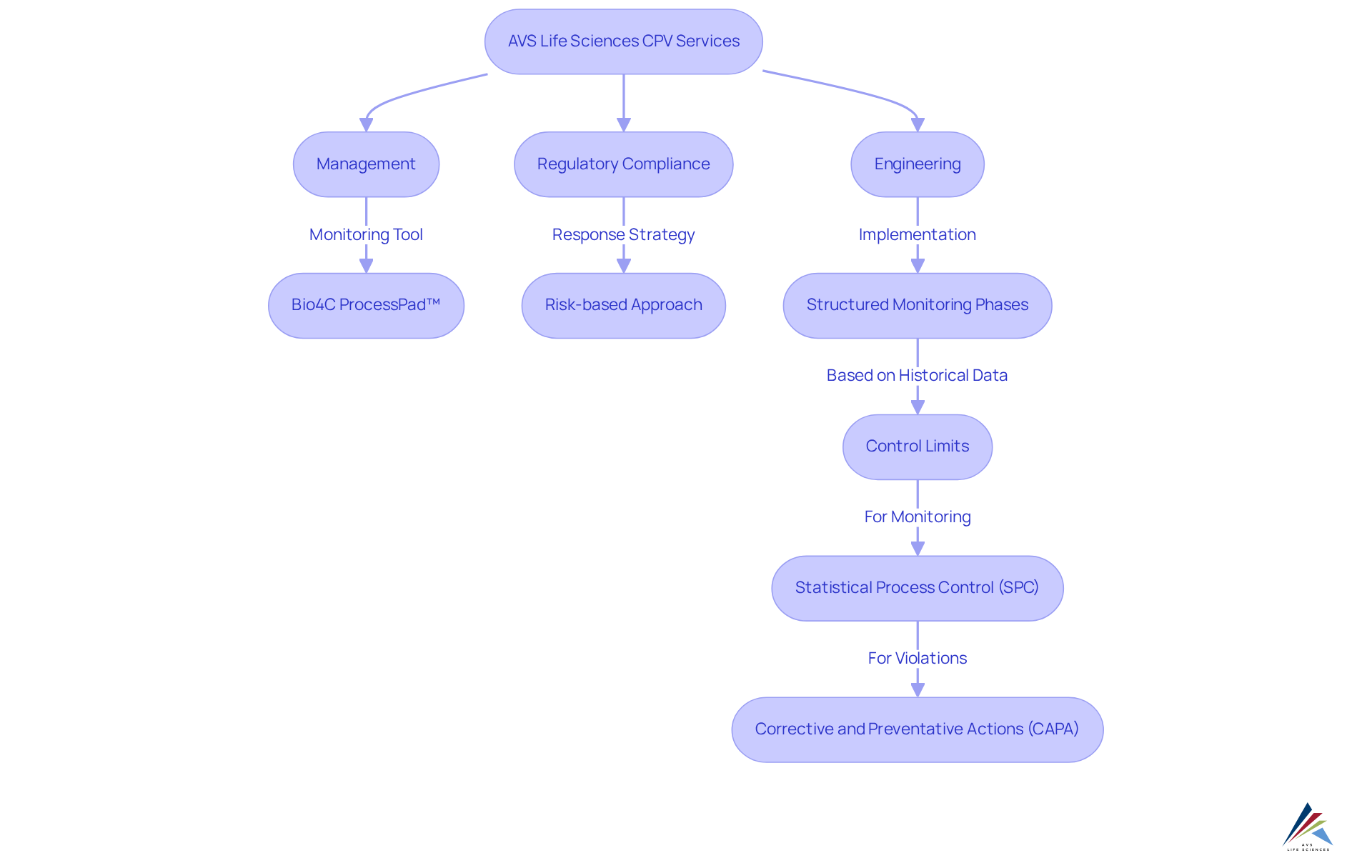
AVS Life Sciences: Comprehensive Solutions for Continued Process Verification (CPV)
AVS Life Sciences offers a comprehensive suite of services meticulously designed to support continued process verification (cpv) in the pharmaceutical sector. Their extensive expertise in management, regulatory compliance, and engineering empowers clients to implement effective continued process verification (cpv) strategies that align with Good Manufacturing Practices (GMP) and other regulatory standards. By focusing on innovation and customer-centric solutions, AVS Life Sciences enhances operational control and product quality throughout the manufacturing lifecycle.
The impact of continued process verification (cpv) on product standards is profound, as it ensures that critical parameters (CPPs) are consistently monitored and regulated. This ongoing verification process is vital for , purity, quality, and safety of pharmaceutical products. Recent trends in continued process verification (cpv) highlight the importance of integrating automated software tools, such as Bio4C ProcessPad™, which facilitate streamlined monitoring and reporting, thereby enhancing efficiency and accuracy in data management.
Successful strategies of continued process verification (cpv) are exemplified through various case studies, illustrating the significance of structured monitoring phases and the establishment of control limits derived from historical data. For instance, one case study demonstrated that employing a unified data software environment significantly reduced errors and improved trend identification, ultimately enhancing overall performance.
Expert insights emphasize the necessity of a risk-based approach to continued process verification (cpv), indicating that not all signals necessitate equivalent responses. This tailored response strategy amplifies the effectiveness of continued process verification (cpv) programs, ensuring timely and scientifically sound decisions that maintain operational continuity. As the pharmaceutical sector evolves, the commitment of AVS Life Sciences to excellence in continued process verification (cpv) establishes them as a dependable partner in navigating the complexities of product development and regulatory compliance.
Understanding the Purpose of Continued Process Verification (CPV)
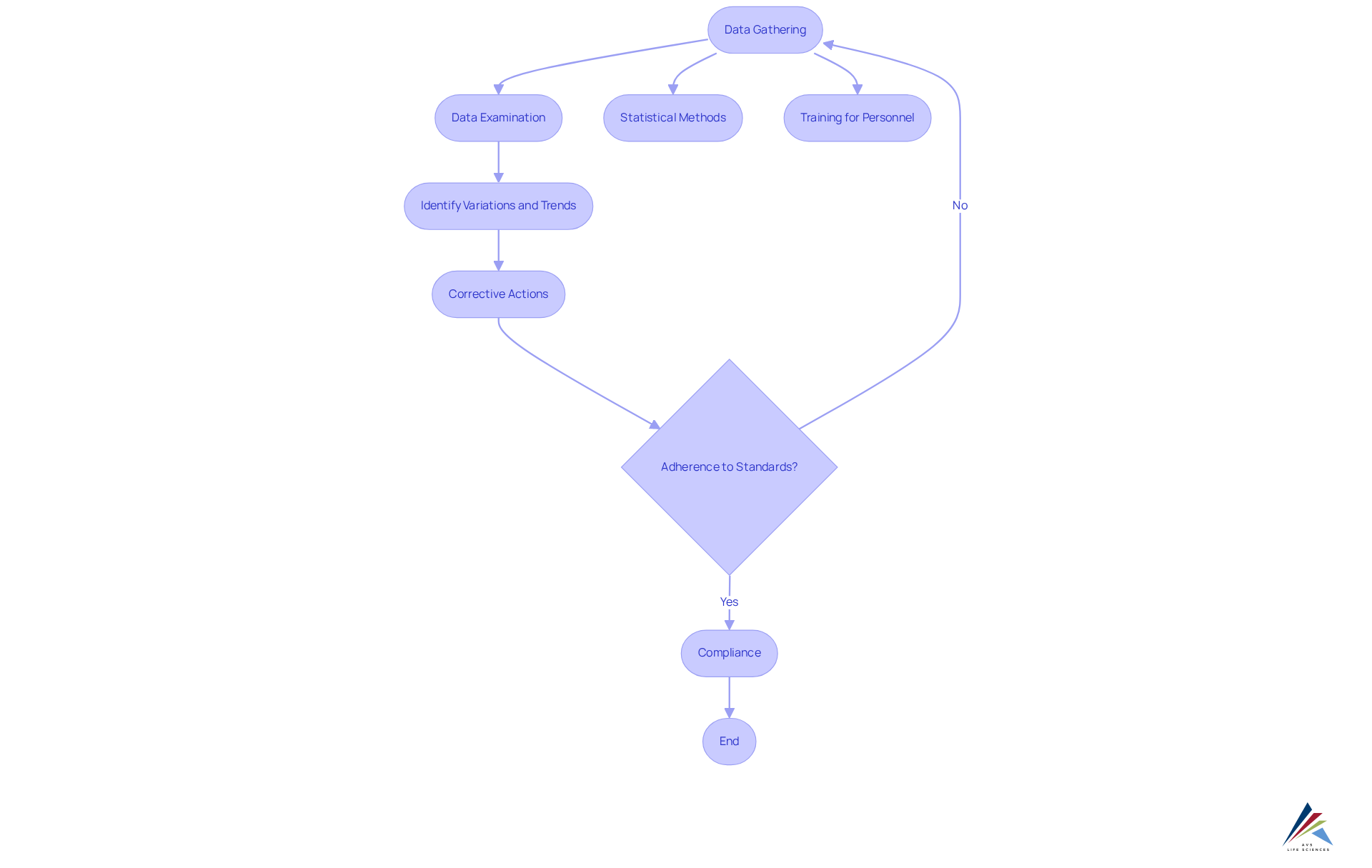
Continued process verification (cpv) serves as a vital framework for monitoring and ensuring that pharmaceutical manufacturing processes remain consistently controlled. Its primary aim is to deliver continuous assurance that products meet established standards throughout their lifecycle, in accordance with GXP and FDA regulations. By systematically gathering and examining data, CPV identifies variations and trends that may influence product standards, enabling prompt corrective actions and compliance with regulatory requirements, including Standard Operating Procedures (SOPs) and Data Integrity protocols.
The significance of continued process verification (cpv) in ensuring the excellence of products is paramount. It enhances Quality Management Systems (QMS) by seamlessly integrating with existing methods, thereby improving product standards and adherence to guidelines. Statistical methods, such as control charts and capability analyses, are employed to oversee critical parameters (CPPs) and essential quality attributes (CQAs), ensuring that any deviations are swiftly managed.
The advantages of continued process verification (cpv) extend beyond mere compliance; they include improved efficiency and reduced errors through automated reporting systems. For instance, automated CPV reporting can yield real-time insights, significantly enhancing decision-making activities. Moreover, the implementation of inline and online sensors facilitates continuous monitoring of critical parameters like pH and temperature, ensuring that manufacturing processes remain within specified limits.
Evidence from numerous studies indicates that organizations that utilize continued process verification (cpv) experience significant enhancements in assurance of standards. By maintaining a validated state throughout the product lifecycle, as mandated by the FDA and EU GMP Annex 15, continued process verification (cpv) not only safeguards product integrity but also fosters a culture of continuous improvement. This proactive approach is crucial for preventing production losses due to manufacturing errors, ultimately leading to enhanced patient safety and product efficacy.
Training for personnel is also essential when incorporating CPV systems, as it ensures that staff possess the necessary knowledge to operate efficiently within this framework, aligning with AVS Life Sciences' commitment to thorough management and .
In summary, continued process verification (cpv) is indispensable for ensuring product excellence in pharmaceutical manufacturing. Its systematic approach to data gathering and examination, coupled with robust statistical techniques, enables organizations to uphold high standards of excellence and compliance, thereby reinforcing their dedication to superiority in the life sciences field.
Navigating Regulatory Requirements for Continued Process Verification (CPV)
Navigating the regulatory landscape for continued process verification (CPV) requires a comprehensive understanding of the guidelines established by regulatory authorities like the FDA and EMA. These guidelines mandate that manufacturers incorporate continued process verification (CPV) within their validation lifecycle, highlighting the importance of establishing critical parameters (CPPs) and critical quality attributes (CQAs). Consistent data evaluation is imperative, as it empowers organizations to proactively identify trends and deviations, ensuring that procedures remain within established control limits. Furthermore, comprehensive documentation is a fundamental requirement, as it bolsters transparency and traceability throughout the manufacturing process.
To illustrate the effectiveness of continued process verification (CPV), statistics reveal that organizations implementing robust continued process verification (CPV) programs attain significantly to regulatory standards. A transformative case study from AVS Life Sciences exemplifies this, showcasing how the company aided a leading biotechnology firm in upgrading their manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. Throughout this upgrade, challenges such as anomalies in test results were uncovered, including overlooked issues with barcode scanner installations. This collaboration not only ensured compliance with regulatory standards but also enhanced the client's operational efficiency, enabling them to concentrate on developing medicines for serious diseases.
The insights garnered from this experience prompted the QC laboratory team and Quality team to evaluate their operational methods, fostering a culture of continuous improvement. This adaptability to changes in the manufacturing environment not only meets regulatory expectations but also leads to elevated product standards and enhanced patient safety. As emphasized by the FDA, 'continued process verification (CPV) is vital for sustaining a state of control during the manufacturing cycle,' highlighting the critical nature of integrating CPV into assurance strategies.

Implementing Effective Data Collection and Monitoring Strategies in CPV
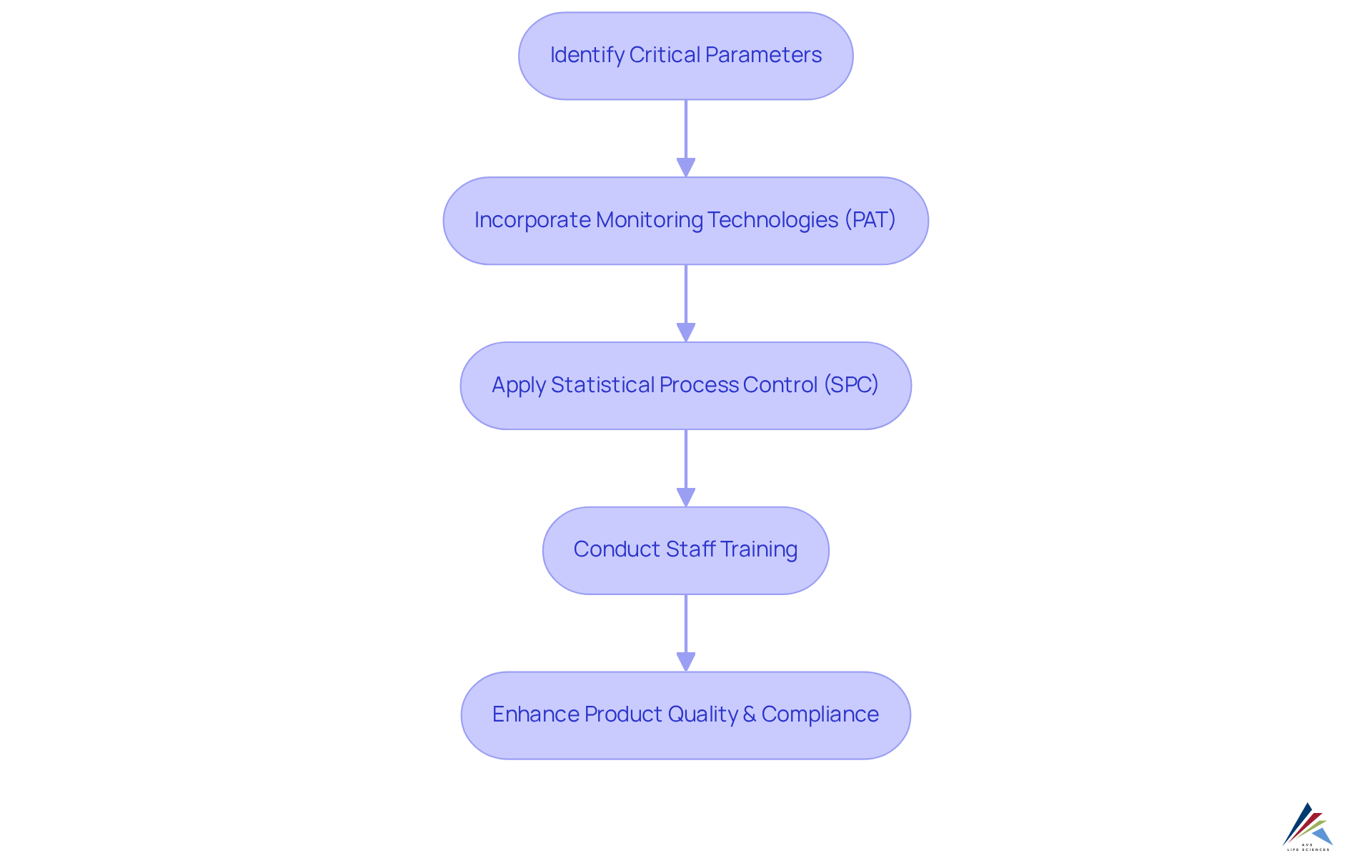
Implementing effective data collection and monitoring strategies in the continued process verification (CPV) is crucial for maintaining product quality and ensuring compliance with regulatory standards such as GXP and FDA regulations. Organizations must first identify critical parameters that significantly influence product outcomes. The incorporation of , particularly Process Analytical Technology (PAT), facilitates real-time data collection and analysis, delivering instant insights into performance.
Furthermore, the application of Statistical Process Control (SPC) techniques, including instruments like control charts and capability indices, is vital for the early identification of trends and deviations, enabling proactive modifications to maintain control.
Regular training for staff on data management practices is essential to ensure the consistency and accuracy of data collection, ultimately reinforcing robust continued process verification (CPV) initiatives and aligning with comprehensive quality management practices in the life sciences sector.
Conducting Risk Assessment and Management in Continued Process Verification
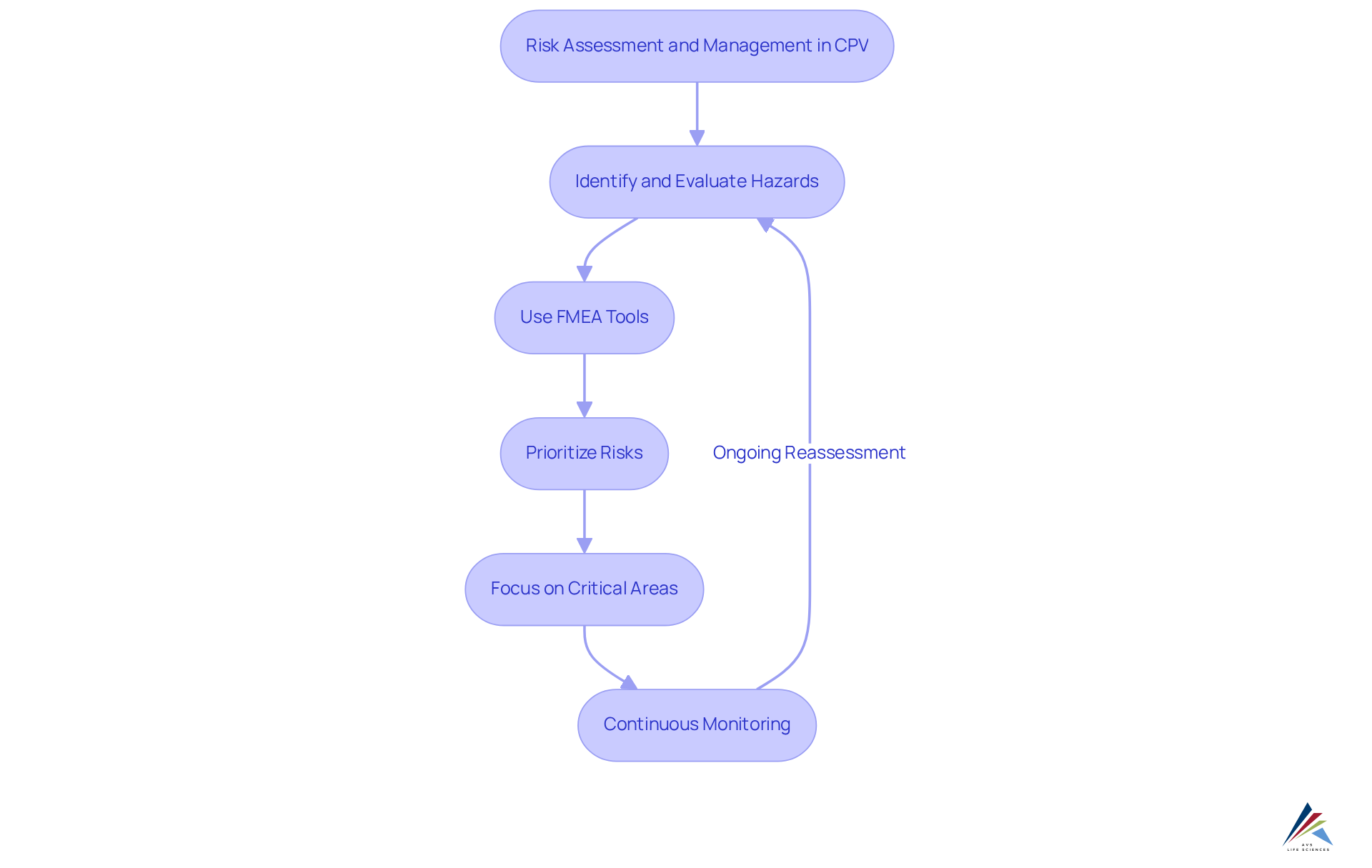
Performing risk assessment and management in the continued process verification (cpv) is critical for identifying and evaluating potential hazards linked to manufacturing operations. Organizations must employ tools such as Failure Mode and Effects Analysis (FMEA) to systematically assess risks associated with critical process parameters. By prioritizing these risks based on their potential impact on product quality, companies can effectively concentrate their efforts on continued process verification (cpv) in the most vital areas.
Continuous monitoring and regular reassessment of risks are essential to adapt to changes in the manufacturing environment and ensure ongoing compliance. Moreover, integrating a comprehensive process checklist for Computer System Validation (CSV) significantly enhances risk management strategies. This checklist should encompass key stages such as:
- Planning
- Defining User Requirement Specifications (URS)
- Conducting Installation, Operational, and Performance Qualification (IQ, OQ, PQ) testing
By adhering to these organized phases, organizations can ensure that their systems not only meet regulatory compliance but also uphold the highest standards of assurance in the life sciences sector.
Integrating Continued Process Verification with Quality Management Systems
Incorporating continued process verification (CPV) with Quality Management Systems (QMS) is crucial for establishing a unified assurance framework. This integration enables smooth data transfer between management procedures and continued process verification (CPV) activities, facilitating that improves operational efficiency. By aligning continued process verification (CPV) metrics with QMS goals, organizations can ensure that standards are consistently achieved, particularly in adherence to GXP regulations and FDA guidelines.
Companies employing a management system during product recalls have reported substantial enhancements in recovery methods, highlighting the efficacy of this integration. Furthermore, this integration facilitates the documentation and reporting obligations required by regulatory bodies, improving overall adherence. It simplifies the management of essential performance attributes (CQAs) and crucial parameters (CPPs), enabling organizations to quickly tackle deviations and uphold compliance.
AVS Life Sciences provides comprehensive regulatory and quality solutions across biopharmaceuticals, medical devices, and nutraceuticals, ensuring that organizations can effectively manage quality in a virtual environment while adhering to best practices in documentation, standard operating procedures (SOPs) development, and data integrity.

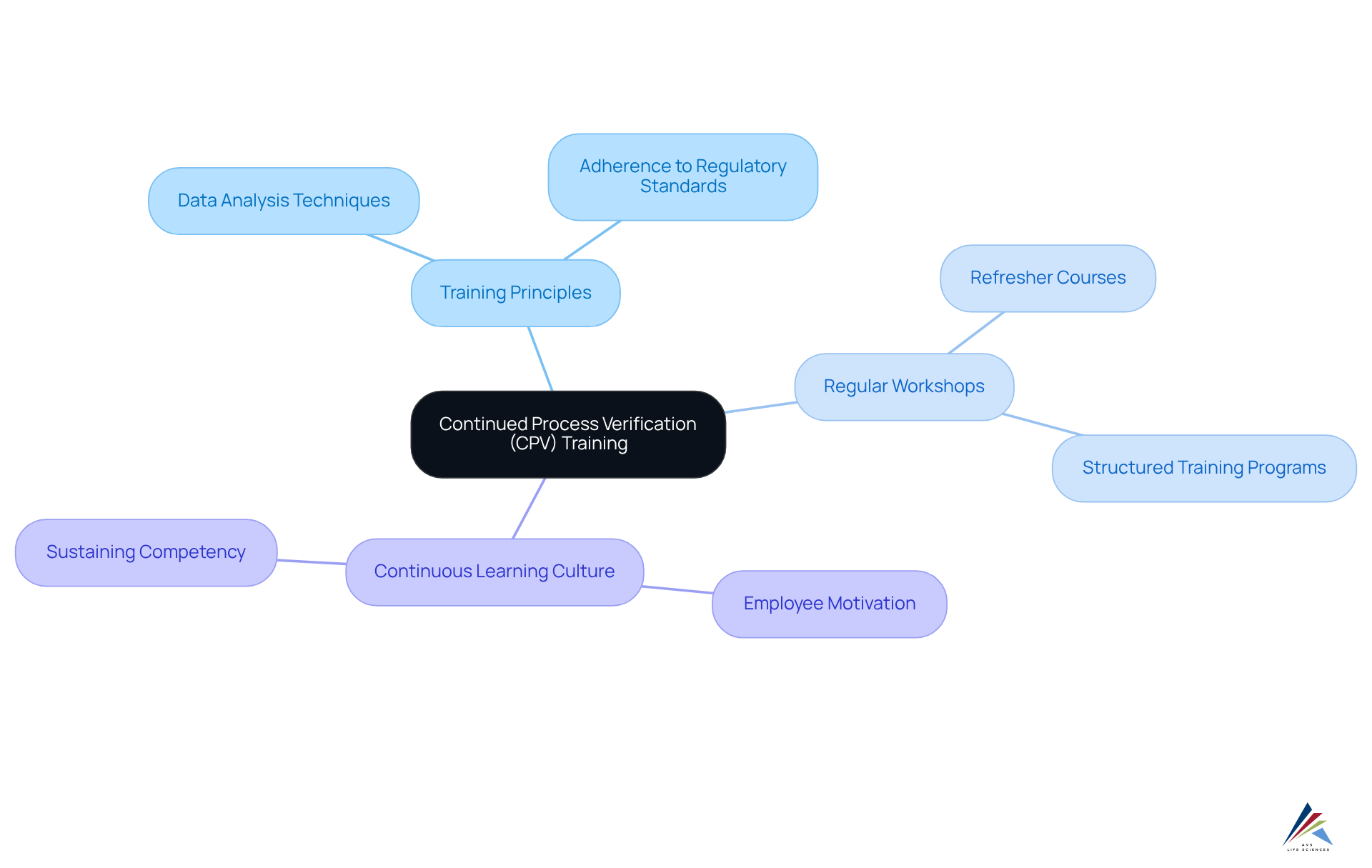
Developing Training and Competency Programs for Continued Process Verification
Establishing robust training and skill development programs for continued process verification (CPV) is essential for equipping staff to effectively manage CPV activities. Organizations must develop comprehensive training programs that encompass the principles of continued process verification (CPV), data analysis techniques, and adherence to regulatory standards.
Regular workshops and refresher courses play a vital role in sustaining staff competency, ensuring they remain knowledgeable about the and practices. For example, companies that have instituted structured training programs report notable enhancements in employee performance and confidence in executing continued process verification (CPV) tasks.
Furthermore, fostering a culture of continuous learning not only motivates employees to actively participate in continued process verification (CPV) initiatives but also enhances overall operational efficiency. Data indicates that organizations with well-trained personnel encounter fewer regulatory challenges and enjoy greater stability in operations, which underscores the critical importance of skill development in continued process verification (CPV).
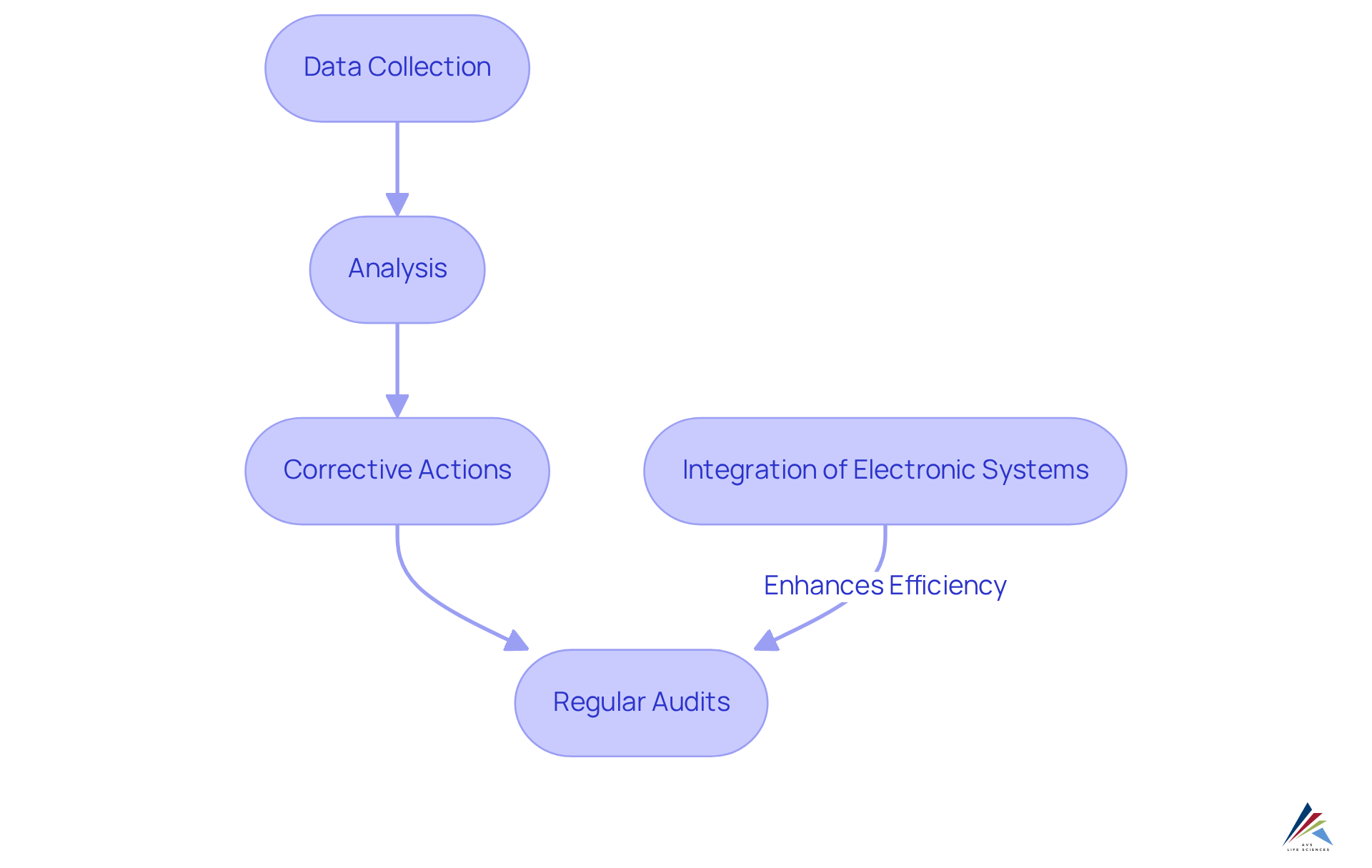
Ensuring Robust Documentation and Reporting Practices in CPV
Thorough in continued process verification (CPV) are essential for upholding regulations and ensuring standards assurance. Organizations must implement standardized documentation procedures that comprehensively capture all activities related to continued process verification (CPV), including:
- Data collection
- Analysis
- Corrective actions
Regular audits of these documentation practices are vital for identifying areas needing improvement, ensuring that records remain complete and accurate. The integration of electronic documentation systems significantly enhances operational efficiency, allowing for streamlined access to records during regulatory inspections. This shift not only supports audit readiness but also aligns with regulatory expectations. Firms that fail to maintain adequate documentation risk receiving observations during audits.
Efficient reporting systems, such as those employing statistical control (SPC) tools, can offer real-time insights into performance, further enhancing compliance efforts. By prioritizing these practices, organizations can foster a culture of quality and continuous improvement within their continued process verification (CPV) systems.
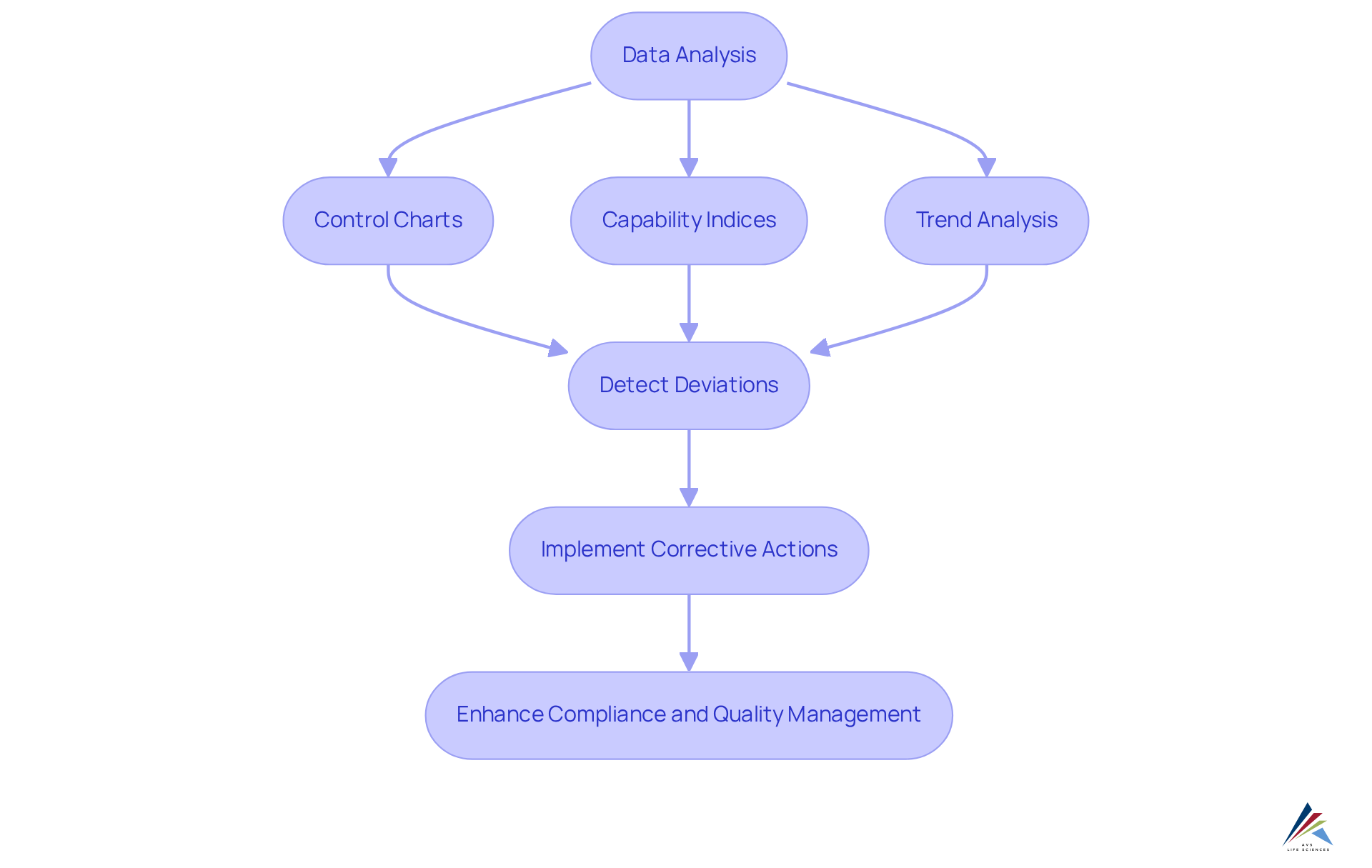
Utilizing Statistical Analysis and Trending in Continued Process Verification
Employing statistical analysis and trending in continued process verification (cpv) enables organizations to effectively oversee performance and detect deviations from anticipated results. Techniques such as control charts, capability indices, and trend analysis yield valuable insights into stability and variability, which are essential for maintaining compliance with GXP and FDA regulations. Regulatory authorities such as the FDA, EMA, and ICH mandate continued process verification (cpv), underscoring its necessity in the pharmaceutical industry. By regularly analyzing data, organizations can detect trends early and implement corrective actions before issues escalate. This proactive approach enhances data integrity and reinforces through effective Standard Operating Procedures (SOPs) and documentation practices.
Control chart limits should be established only when at least 30 lots are available, following the Levey-Jennings approach, to ensure effective monitoring practices. Moreover, evaluating normality in capability estimates is essential for precise statistical analysis. Incorporating advanced statistical tools, such as statistical process control (SPC) software, significantly improves the accuracy and efficiency of data analysis, facilitating informed decision-making. As the regulatory environment continues to evolve, utilizing these statistical techniques not only meets regulatory demands but also fosters ongoing enhancement in product standards and operational effectiveness. This commitment strengthens the role of continued process verification (cpv) in lifecycle management and continuous control, emphasizing AVS Life Sciences' dedication to expert solutions in GMP compliance and assurance.
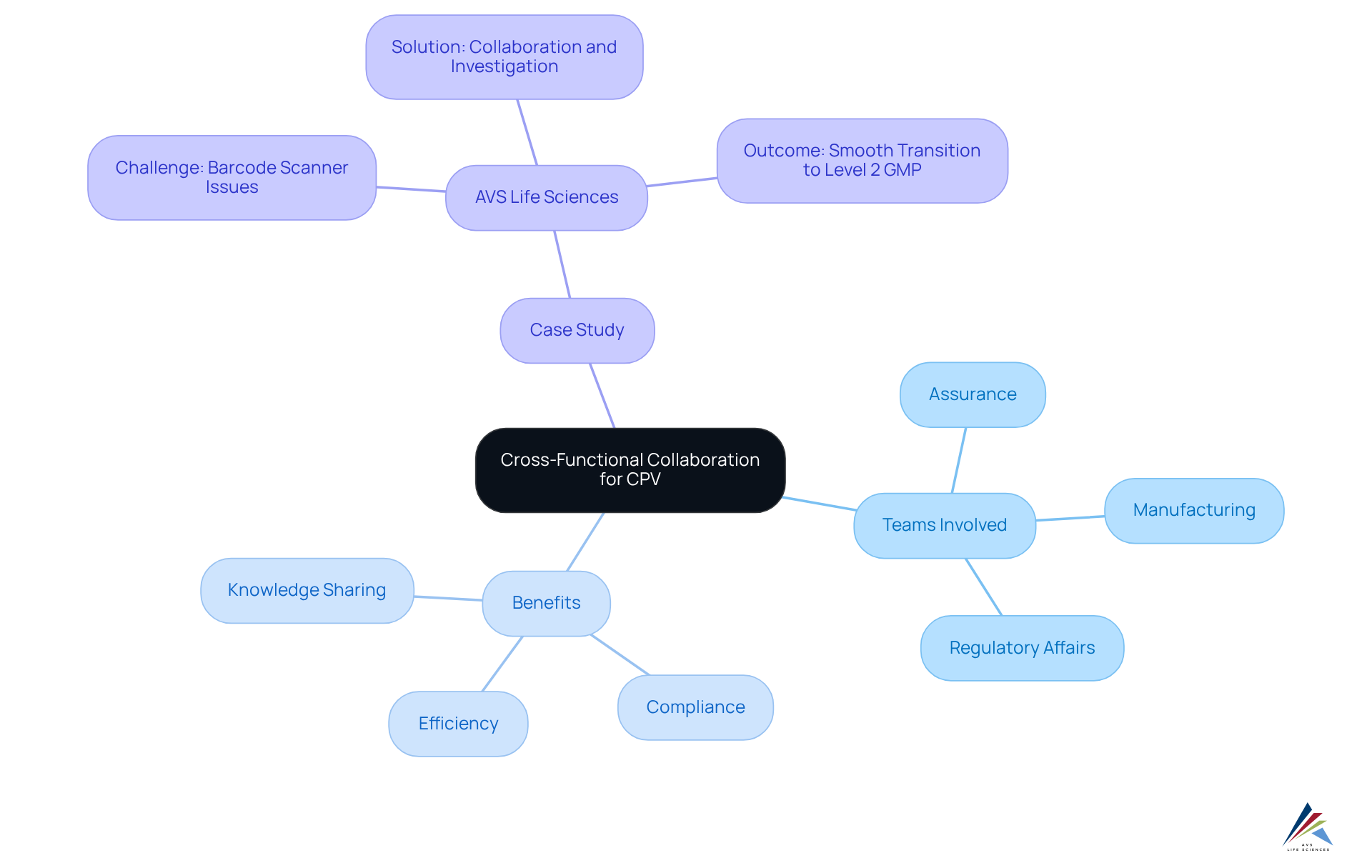
Fostering Cross-Functional Collaboration for Effective Continued Process Verification
Successful continued process verification (cpv) relies on fostering cross-functional cooperation among teams from assurance, manufacturing, and regulatory affairs. Engaging diverse departments significantly enhances the effectiveness of continued process verification (CPV) efforts by promoting open communication and shared ownership of the outcomes of CPV.
Regular cross-departmental meetings and collaborative projects facilitate knowledge sharing, ensuring that all stakeholders are aligned with the objectives of continued process verification (cpv). This collaborative approach not only strengthens control over procedures but also cultivates a robust quality culture within the organization.
Notably, organizations prioritizing teamwork in continued process verification (CPV) initiatives have reported substantial improvements in compliance and efficiency, with approximately 90% of products now having a continued process verification (CPV) plan in place, a remarkable increase from just 30% two years ago.
A transformative case study from AVS Life Sciences effectively illustrates this point. The company assisted a leading biotechnology firm in upgrading their manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. During this project, challenges emerged, including anomalies in test results due to barcode scanner cameras being installed upside down, which led to incorrect readings. This oversight was addressed through thorough investigation and , enabling the client to concentrate on developing medicines for serious diseases while AVS Life Sciences ensured a smooth and compliant transition in accordance with industry standards.
The lessons learned during the upgrade process prompted a reevaluation of business processes, ultimately enhancing the reliability of test results and fostering open discussions about team responsibilities. This reinforces the value of a united approach in overcoming challenges and driving innovation.
Additionally, as Patrick Lencioni noted, "Trust is the glue that binds a team together," underscoring the importance of trust in fostering collaboration among teams. This commitment to teamwork is essential for navigating the complexities of continued process verification (cpv) during execution.
Conclusion
The essence of continued process verification (CPV) lies in its ability to ensure that pharmaceutical manufacturing processes consistently meet established quality standards throughout the product lifecycle. By implementing robust CPV strategies, organizations can not only comply with regulatory requirements but also enhance product quality and operational efficiency.
This article has explored ten key strategies for achieving success in continued process verification:
- Leveraging advanced data collection and monitoring technologies
- Fostering cross-functional collaboration among teams
- Maintaining compliance
- Driving continuous improvement
- Integrating statistical analysis
- Supporting effective training programs
- Establishing a culture dedicated to quality assurance
- Prioritizing risk management
- Utilizing real-time feedback mechanisms
- Engaging stakeholders throughout the process
As the pharmaceutical industry continues to evolve, the importance of continued process verification cannot be overstated. Organizations are encouraged to adopt these best practices and stay ahead of regulatory demands. By prioritizing CPV, companies not only safeguard product integrity and patient safety but also position themselves as leaders in quality management within the life sciences sector.
Frequently Asked Questions
What is Continued Process Verification (CPV)?
Continued Process Verification (CPV) is a framework used in pharmaceutical manufacturing to monitor and ensure that processes remain consistently controlled, delivering continuous assurance that products meet established standards throughout their lifecycle.
How does AVS Life Sciences support CPV?
AVS Life Sciences provides a comprehensive suite of services that includes management, regulatory compliance, and engineering expertise to help clients implement effective CPV strategies that align with Good Manufacturing Practices (GMP) and regulatory standards.
Why is CPV important in the pharmaceutical sector?
CPV is crucial as it ensures the identity, purity, quality, and safety of pharmaceutical products by consistently monitoring critical parameters (CPPs) and facilitating timely corrective actions to comply with regulatory requirements.
What role do automated tools play in CPV?
Automated software tools, such as Bio4C ProcessPad™, streamline monitoring and reporting processes, enhancing efficiency and accuracy in data management during CPV.
What are some successful strategies for implementing CPV?
Successful CPV strategies involve structured monitoring phases, establishing control limits based on historical data, and utilizing unified data software environments to reduce errors and improve trend identification.
What is a risk-based approach to CPV?
A risk-based approach to CPV tailors responses to different signals, ensuring that not all variations require the same level of attention, which enhances the effectiveness of CPV programs.
How does CPV improve Quality Management Systems (QMS)?
CPV enhances QMS by integrating with existing methods, improving product standards, and ensuring compliance with guidelines through systematic data gathering and statistical methods.
What are the benefits of automated CPV reporting?
Automated CPV reporting provides real-time insights, significantly improving decision-making and reducing errors, which contributes to overall operational efficiency.
What regulatory requirements must be met for CPV?
Manufacturers must incorporate CPV within their validation lifecycle as mandated by regulatory authorities like the FDA and EMA, ensuring consistent data evaluation and comprehensive documentation for transparency and traceability.
Can you provide an example of successful CPV implementation?
A case study from AVS Life Sciences demonstrated how they helped a biotechnology firm upgrade their manufacturing facility while identifying and resolving anomalies, leading to enhanced compliance and operational efficiency.
Why is training important for CPV systems?
Training ensures that personnel have the necessary knowledge to operate effectively within CPV frameworks, aligning with regulatory adherence and management solutions in the life sciences sector.
