10 Key Strategies for cgmp Medical Compliance Success

Overview
This article outlines ten key strategies for achieving compliance with current Good Manufacturing Practices (cGMP) in the medical field. It emphasizes the critical importance of a comprehensive Quality Management System (QMS), personnel training, and effective documentation. Organizations that implement robust compliance measures experience significant benefits, including:
- Improved operational efficiency
- Reduced non-compliance incidents
- Enhanced product quality
These outcomes ultimately foster a culture of excellence within the life sciences sector. By understanding these strategies, compliance officers can navigate the complexities of regulatory requirements and drive their organizations towards success.
Introduction
In the highly regulated landscape of pharmaceuticals, maintaining compliance with current Good Manufacturing Practices (cGMP) is not merely a requirement—it is a commitment to quality and safety. Organizations striving to meet these stringent standards often find the path to success daunting, filled with complex regulations and evolving practices. This article explores ten key strategies designed to empower compliance officers and pharmaceutical companies in navigating the intricacies of cGMP compliance effectively.
What essential practices can transform compliance efforts from mere obligation into a robust framework for operational excellence?
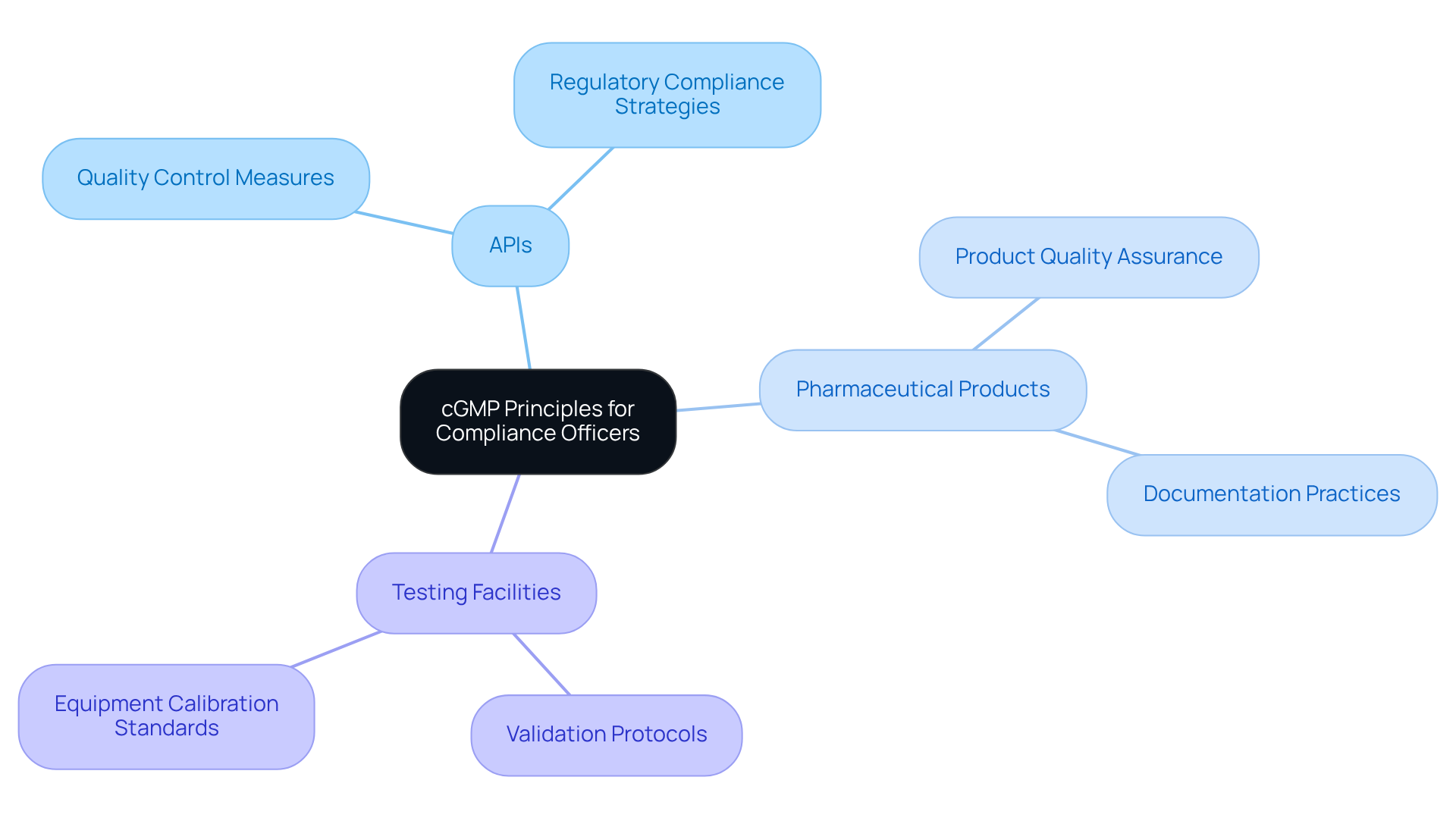
AVS Life Sciences: Comprehensive Guidance on cGMP Principles for Compliance Officers
AVS Life Sciences provides comprehensive guidance on cgmp medical practices specifically designed for regulatory officers. Their expertise in CGMP medical evaluations encompasses critical sectors such as APIs, pharmaceutical products, and testing facilities, ensuring that organizations can effectively implement current CGMP medical principles essential for maintaining product quality and regulatory compliance.
By leveraging AVS's insights, compliance officers can deepen their understanding of the complexities of cgmp medical practices, facilitating their application within their organizations to mitigate risks and enhance operational efficiency. This ultimately transforms GMP facilities and guarantees adherence for biotechnology clients, paving the way for .
Quality Management Systems: Foundation of Effective cGMP Practices
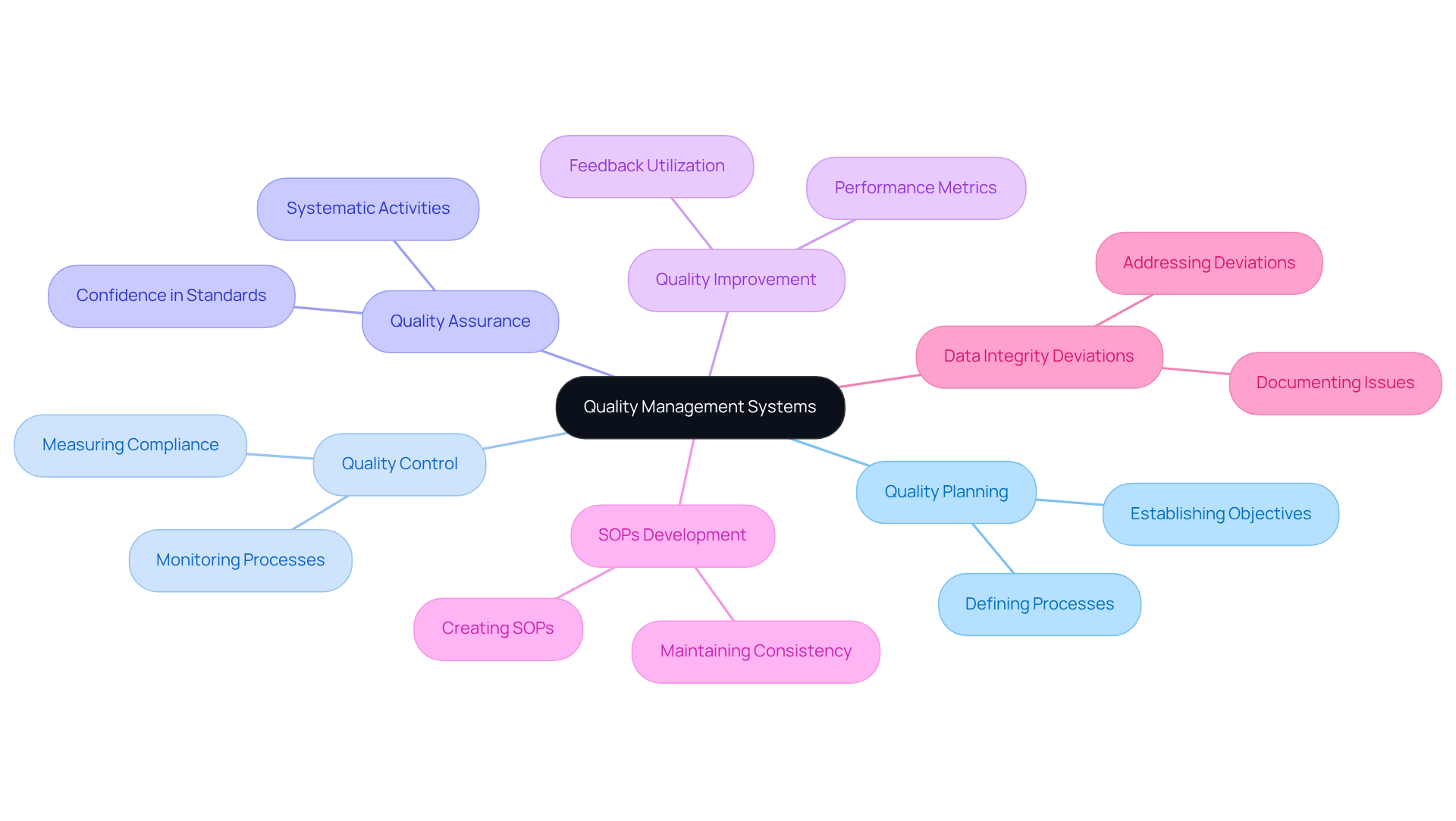
Implementing a Quality Management System (QMS) is imperative for achieving effective cGMP practices. A well-organized QMS offers a systematic approach to managing standards across all operations, ensuring that processes are standardized and comply with regulatory requirements, including GXP and FDA regulations. The key components of an effective QMS include:
- Quality Planning: Establishing clear objectives and processes to meet quality standards.
- Quality Control: Monitoring and measuring processes to ensure they meet specified requirements.
- Quality Assurance: Implementing systematic activities to provide confidence that standards will be fulfilled.
- Quality Improvement: Continuously enhancing processes based on feedback and performance metrics.
- Standard Operating Procedures (SOPs) Development: Creating and maintaining SOPs to ensure consistent excellence practices.
- Data Integrity Deviations: Addressing and documenting any deviations to maintain data integrity.
These components work synergistically to uphold high standards in product manufacturing and safety. Statistics reveal that organizations employing experience a 25% higher customer retention rate and a 40% reduction in customer complaints. Recent advancements in QMS, particularly the integration of automated solutions and real-time monitoring, further enhance adherence and operational efficiency within the pharmaceutical industry. Specialist perspectives underscore that a robust QMS not only ensures compliance with cGMP medical standards but also fosters a culture of continuous improvement, ultimately leading to enhanced product standards and safety. AVS Life Sciences stands out as a premier provider of management and regulatory adherence solutions, delivering comprehensive consulting services that address the unique challenges faced by the life sciences sector.
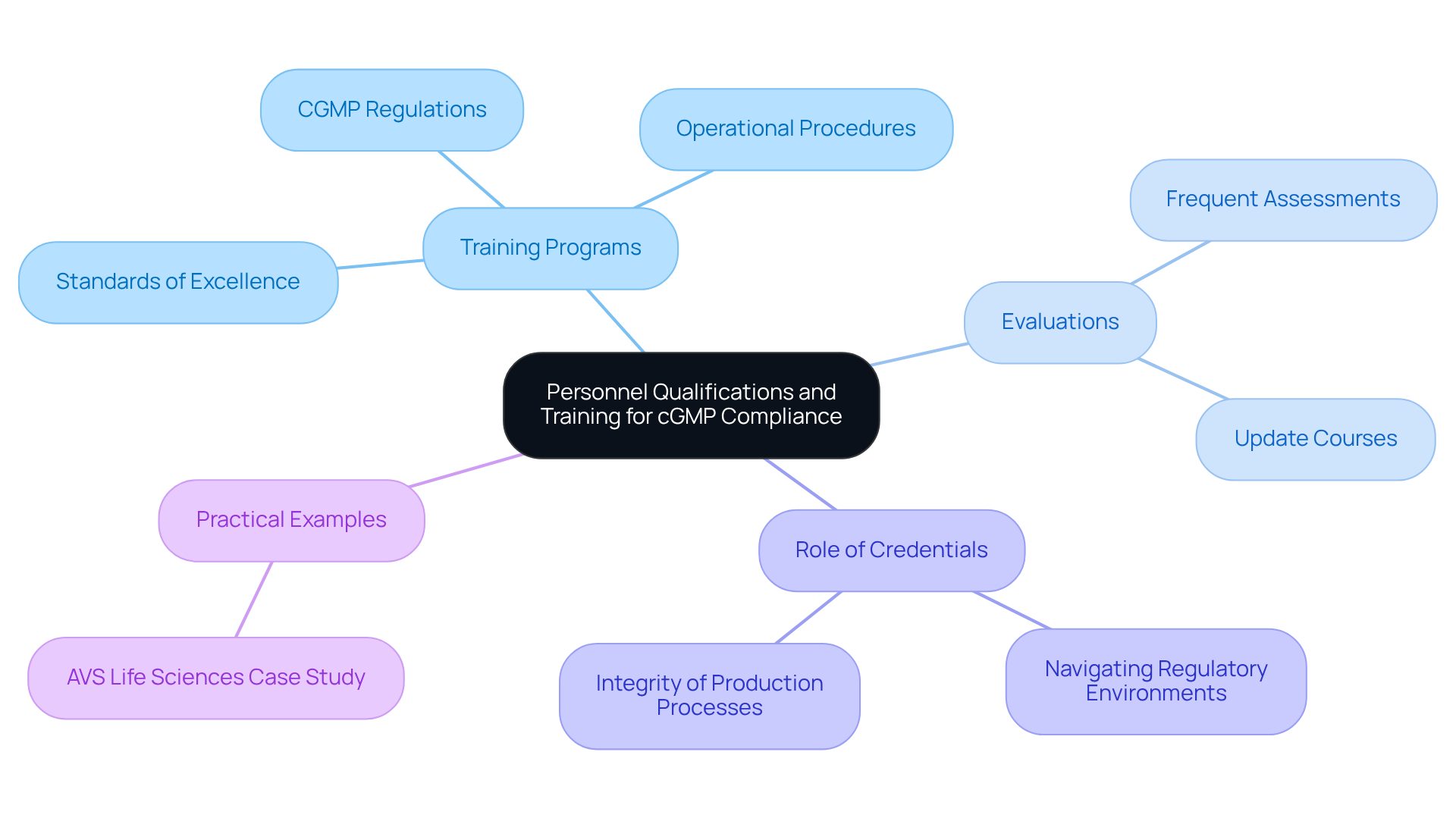
Personnel Qualifications and Training: Ensuring Competence in cGMP Compliance
Ensuring that staff are qualified and properly trained is paramount for upholding compliance with good manufacturing practices. Organizations must implement comprehensive training programs that encompass CGMP medical regulations, operational procedures, and standards of excellence. Frequent evaluations and update courses are essential for maintaining a high standard of proficiency among personnel, thereby reducing the risk of non-compliance and enhancing overall product excellence.
For instance, AVS Life Sciences' successful enhancement of a biotechnology GMP facility exemplifies how effective training programs can lead to substantial improvements in adherence rates and foster a culture of quality and safety. As we approach 2025, the emphasis on personnel training becomes increasingly critical, with effective programs tailored to meet current regulatory requirements being vital for operational success.
Moreover, the credentials of personnel play a crucial role in CGMP medical practices; well-informed workers are better equipped to navigate complex regulatory environments and uphold the integrity of production processes. This was evident in AVS Life Sciences' collaboration with a leading biotechnology firm, where were essential for the successful transition to a Level 2 GMP facility.
Throughout this process, challenges such as anomalies in test results due to improperly installed barcode scanner cameras were identified, leading to significant lessons learned that prompted the QC laboratory team to assess their business processes and enhance their testing protocols.
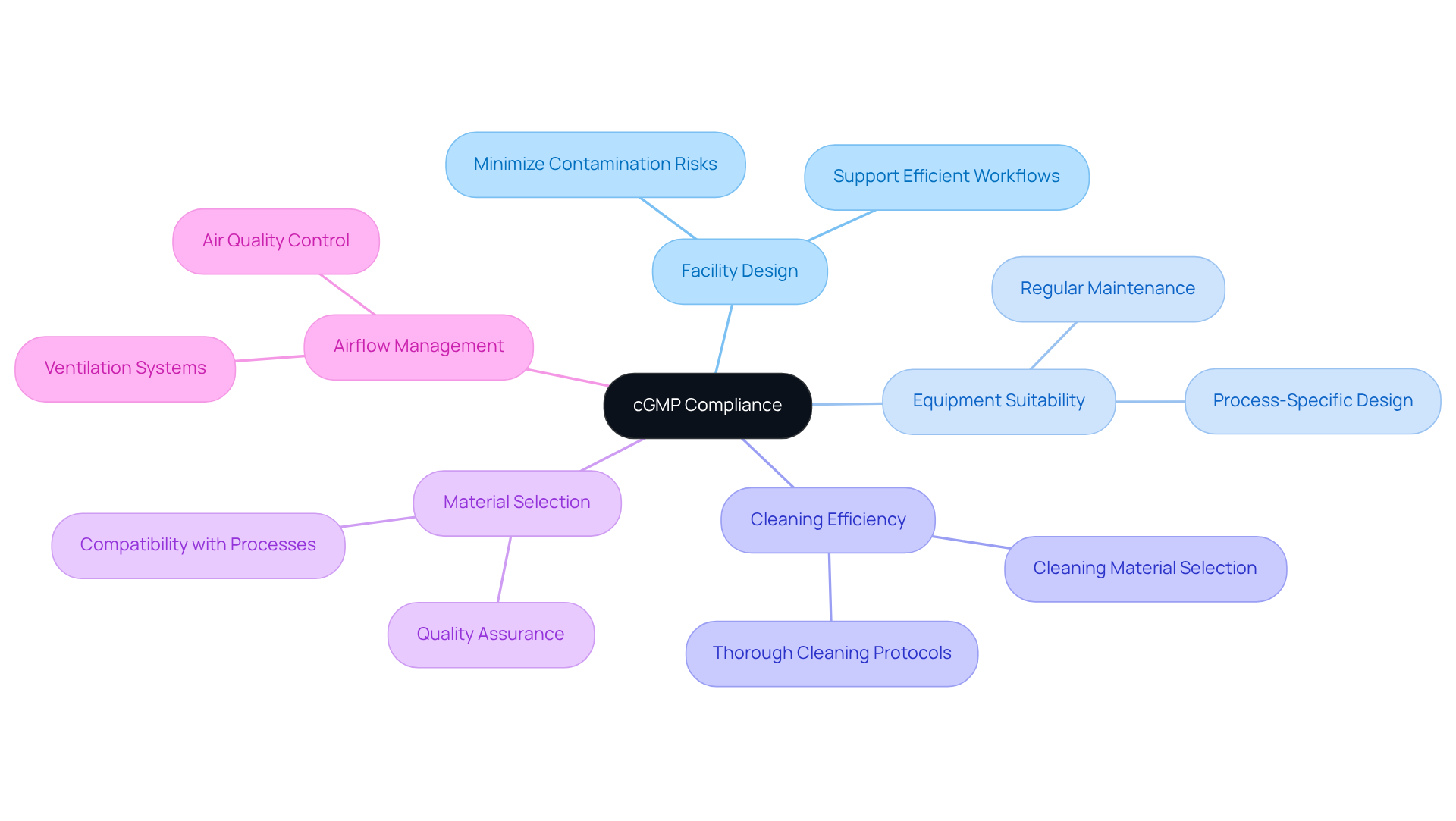
Facility and Equipment Design: Key Considerations for cGMP Compliance
Facility and equipment design are pivotal in ensuring compliance with cGMP standards. Facilities must be strategically designed to minimize contamination risks, facilitate thorough cleaning, and support efficient workflows. Considerations such as layout, materials, and airflow collectively enhance operational efficiency and product safety. Furthermore, equipment must be specifically suited for the intended processes and undergo regular maintenance to guarantee .
Adhering to established design standards not only elevates product quality but also reinforces compliance with guidelines, including cGMP medical, GXP, and FDA regulations, thereby fostering a culture of excellence in manufacturing practices. By implementing these design considerations and adhering to best practices in documentation and standard operating procedures (SOPs), oversight officers can ensure that their facilities not only meet legal standards but also achieve operational excellence.
Control of Materials: Managing Risks in cGMP Operations
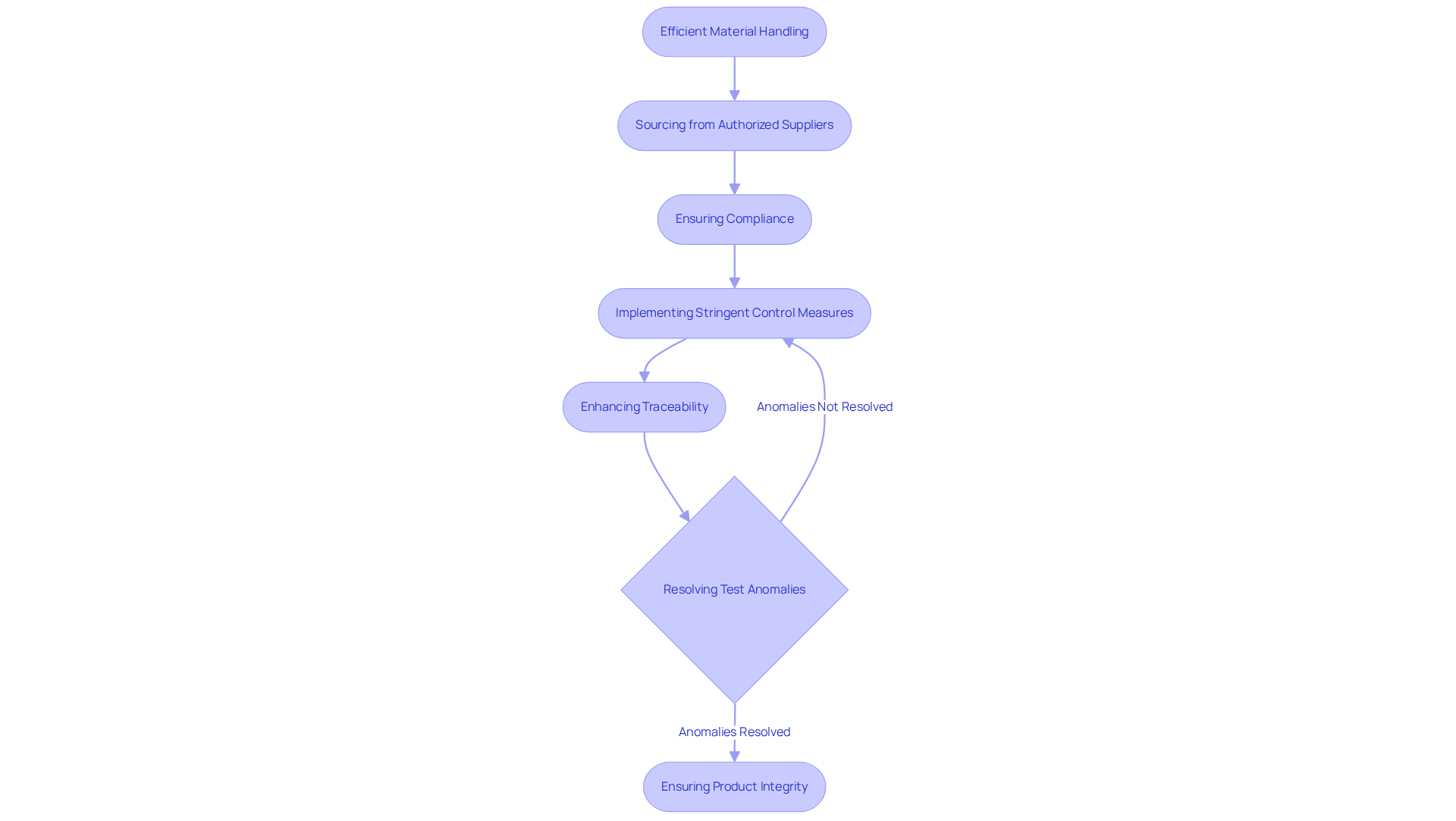
Efficient handling of materials is paramount in cgmp medical practices, as the integrity of raw materials, components, and packaging directly influences product standards. Sourcing from authorized suppliers is essential; it not only ensures compliance with regulatory standards but also mitigates risks related to contamination.
A recent case study by AVS Life Sciences illustrates the successful upgrade of a biotechnology GMP facility, where stringent material control measures were instituted to address contamination risks. During this upgrade, challenges arose, including the necessity for enhanced traceability and the resolution of anomalies in test results. By demonstrating complete traceability and rectifying quality control deficiencies, AVS ensured that only materials compliant with cgmp medical standards were employed in production, thereby safeguarding product integrity.
This proactive approach not only elevates but also enables pharmaceutical producers to focus on their core mission—developing medications that improve patient well-being. The collaboration yielded significant lessons learned, prompting the QC laboratory team to reassess their processes and enhance operational efficiency.
This experience underscores the financial benefits of effective supplier management and the critical importance of continuous training programs tailored to specific roles, reinforcing regulatory adherence and strengthening supplier relationships.
Production and Process Controls: Maintaining Quality in cGMP Practices
Establishing strong production and process controls is essential for maintaining standards in cgmp medical practices. Creating thorough standard operating procedures (SOPs) represents a fundamental step; these documents provide clear instructions for operations, ensuring consistency and adherence. Ongoing oversight and validation studies are crucial to guarantee that processes consistently produce items that meet established standards.
Organizations implementing strict SOPs have observed considerable decreases in deviations and non-conformances, thereby enhancing overall adherence. Continuous training on these SOPs is vital, keeping teams informed about evolving regulations and best practices. By prioritizing effective SOP implementation in cgmp medical environments, organizations can not only meet regulatory requirements but also cultivate a culture of quality that minimizes risks such as contamination and production inconsistencies.
This proactive approach ultimately leads to improved and a stronger reputation in the competitive landscape of the life sciences industry.
Quality Control and Laboratory Testing: Ensuring Compliance with cGMP Standards
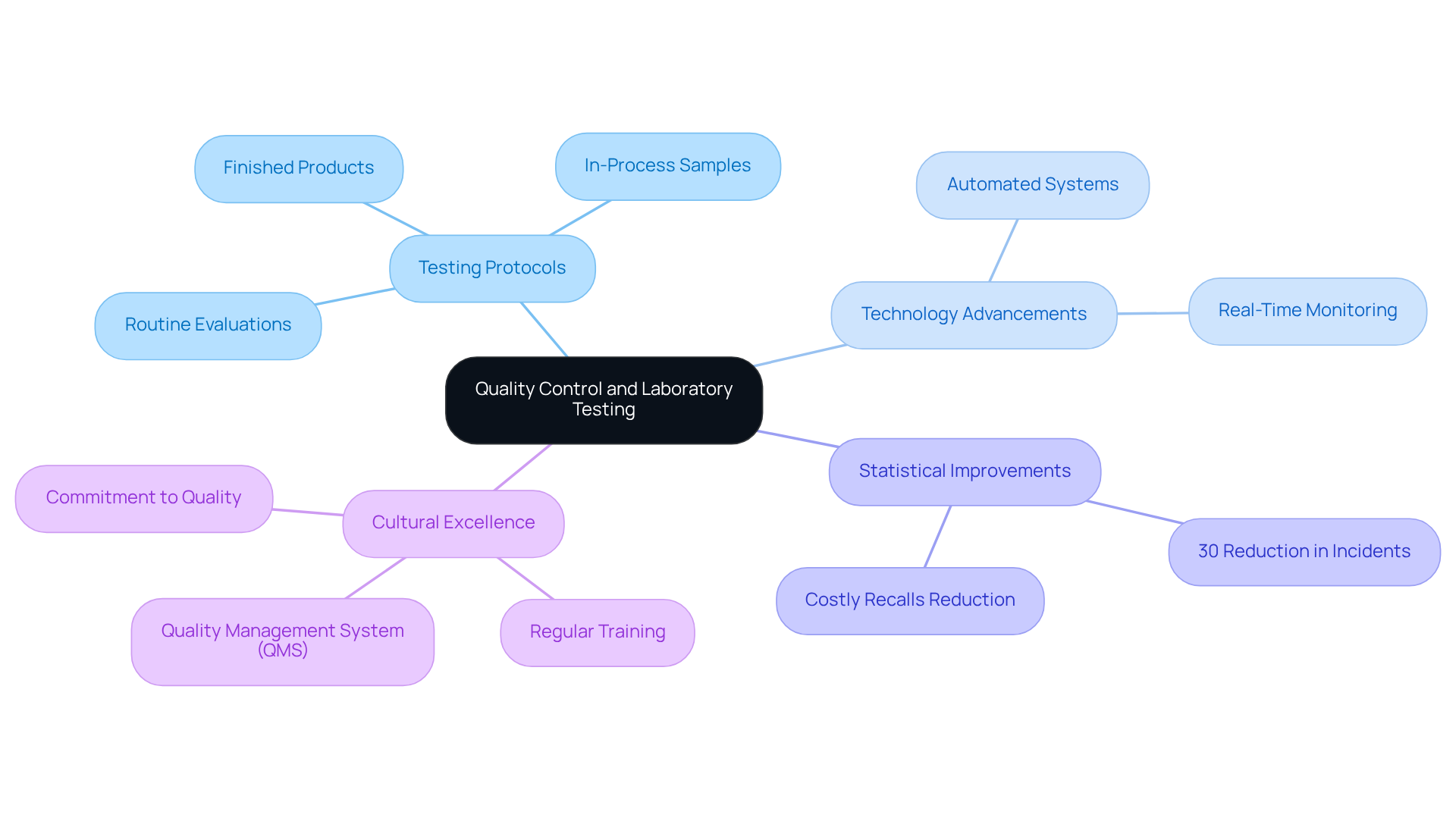
Quality control and laboratory testing are essential pillars of cgmp medical adherence, ensuring that pharmaceutical products consistently meet safety and efficacy standards. Organizations must implement robust testing protocols that encompass routine evaluations of raw materials, in-process samples, and finished products. Recent advancements in laboratory testing technologies, such as automated systems and real-time monitoring, have significantly improved the accuracy and efficiency of compliance testing.
Statistics reveal that adherence to stringent laboratory testing protocols can markedly reduce the incidence of non-compliance issues, which often lead to costly recalls and regulatory penalties. Companies that emphasize control testing report a 30% reduction in compliance-related incidents, underscoring the significance of these practices.
Expert insights highlight the necessity of cultivating a culture of excellence within organizations. This involves regular training for personnel on testing protocols and the implementation of a Quality Management System (QMS) to streamline documentation and enhance traceability. By fostering a commitment to quality, organizations can not only meet compliance standards but also build trust with stakeholders and consumers.
Ultimately, under cgmp medical guidelines transcends mere compliance requirements; it is a critical element of product development that guarantees the provision of safe and effective pharmaceuticals to the market.
Documentation and Record Keeping: Essential for cGMP Compliance
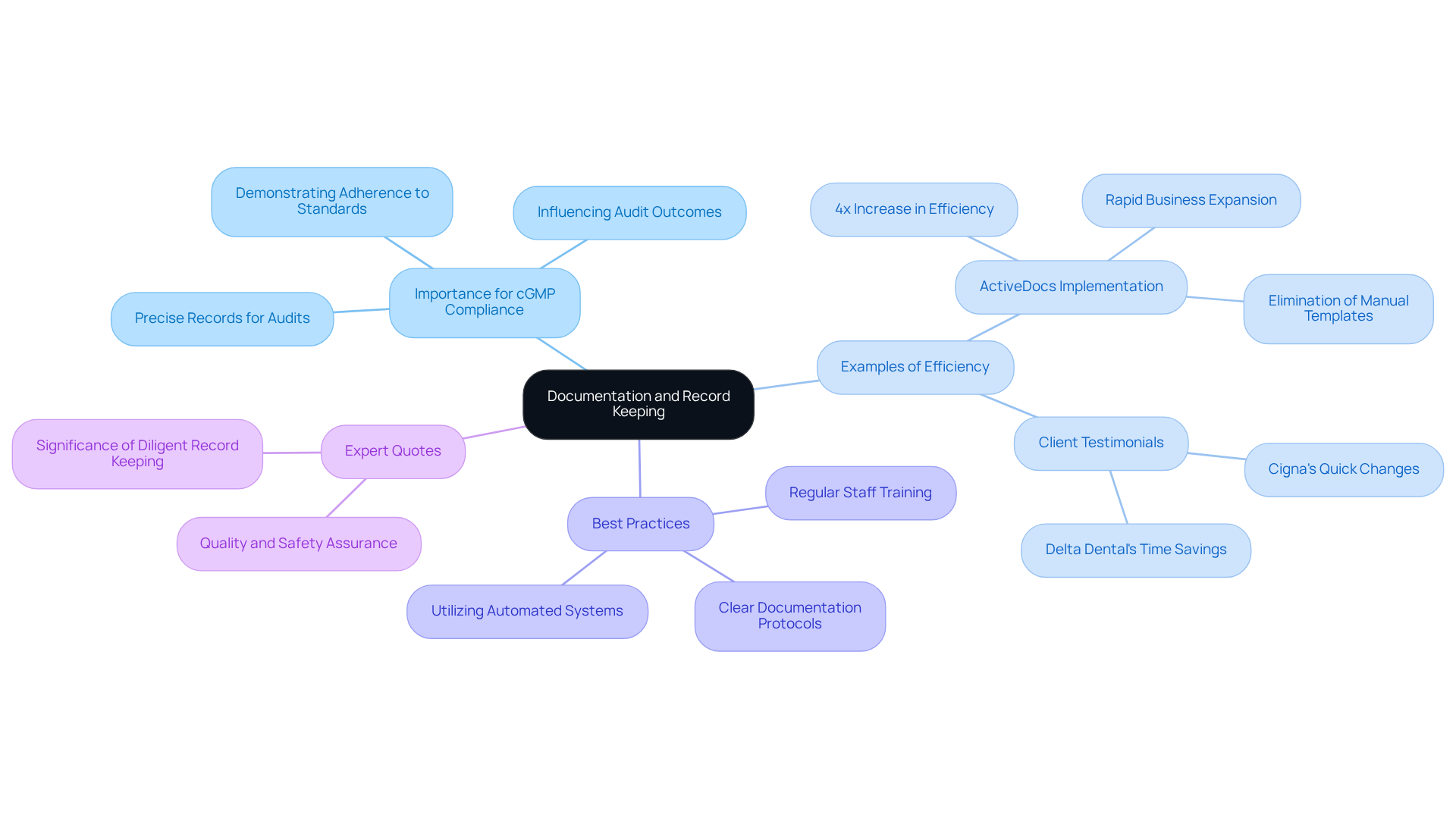
Effective documentation and meticulous record keeping are fundamental to compliance with cgmp medical, especially in 2025, where regulatory scrutiny is intensifying. Organizations must maintain precise and comprehensive records of all processes, including production, quality control, and training activities. These records not only serve as vital evidence during audits and inspections but also demonstrate adherence to cgmp medical standards, significantly influencing audit outcomes.
For instance, companies that have implemented robust document management systems report enhanced efficiency and accuracy in record keeping. A notable example is a major insurance funding provider that achieved a fourfold increase in efficiency through automated documentation processes, allowing for rapid business expansion without additional hires. This efficiency directly leads to enhanced adherence results, as minimizes the risk of mistakes and oversights.
Best practices for record keeping in pharmaceuticals include:
- Establishing clear protocols for documentation at every stage of production.
- Utilizing automated systems to minimize manual entry and enhance accuracy.
- Regularly training staff on the significance of adherence and proper record maintenance.
Quotes from industry leaders underscore the significance of diligent record keeping. One specialist remarked, "Precise documentation is not merely a compliance necessity; it is crucial for guaranteeing product standards and safety."
By emphasizing efficient documentation methods, organizations can not only satisfy compliance standards but also cultivate a culture of quality and adherence that enhances their overall operations.
Continuous Education and Training: Staying Current with cGMP Regulations
Ongoing education and training are vital for organizations aiming to comply with cGMP medical regulations. Implementing continuous training programs that emphasize compliance updates, industry best practices, and emerging trends is crucial. By fostering a culture of continuous learning, companies empower their personnel to stay informed and adept at navigating the complexities of a dynamic regulatory landscape.
Statistics indicate that organizations prioritizing ongoing education witness significant improvements in adherence rates, underscoring the necessity of such initiatives. To effectively implement ongoing training programs, companies should consider the following strategies:
- Develop a structured curriculum that includes regular updates on cGMP regulations and industry standards.
- Utilize a variety of training methods, such as workshops, e-learning modules, and hands-on experiences, to cater to diverse learning styles.
- Encourage employee participation in external training opportunities and industry conferences to expand their knowledge base.
- Foster an environment where inquiries and discussions regarding regulations are welcomed, promoting open communication.
As John C. Maxwell aptly states, "Great leaders surround themselves with smarter individuals," highlighting the importance of investing in employee development. By committing to continuous training, organizations not only enhance their regulatory capabilities but also cultivate a workforce prepared to tackle the challenges of the ever-evolving life sciences sector.
Audits and Inspections: Ensuring Adherence to cGMP Standards
Regular audits and inspections are essential for ensuring adherence to cgmp medical standards. These assessments not only assist organizations in identifying potential regulatory gaps but also reveal areas ripe for enhancement. By acting on audit findings, companies can fortify their compliance frameworks and significantly mitigate the risk of regulatory violations. This proactive strategy not only improves operational efficiency but also fosters a culture of excellence and accountability within the organization.
For instance, AVS Life Sciences recently aided a leading biotechnology company in upgrading its manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. This transformative project encompassed a comprehensive gap analysis and final equipment installation, completed on schedule and within budget. The documentation efforts to ensure full traceability were deemed appropriate by the client’s quality assurance team, underscoring the vital role of quality management in the life sciences sector. During the process, anomalies were discovered in test results that were initially marked as 'Passed' due to improperly installed barcode scanner cameras. This oversight emphasized the necessity for more rigorous testing protocols and led to valuable lessons learned, prompting the QC laboratory and Quality teams to evaluate their business processes for reliability.
Moreover, the biologics market is witnessing rapid growth, with a projected compound annual growth rate of 15% until 2027. This expansion underscores the necessity for organizations to remain vigilant in their adherence to , as the increasing complexity of regulations can lead to heightened scrutiny during inspections. By fostering a culture that prioritizes regular audits, companies can better prepare for inspections and ensure they meet the evolving standards of the industry. To implement effective audits, compliance officers should:
- Establish a regular audit schedule
- Engage cross-functional teams in the audit process
- Utilize audit findings to drive continuous improvement
Conclusion
Implementing effective strategies for cGMP compliance is essential for organizations striving to maintain high standards in the life sciences sector. This article outlines ten key strategies that not only enhance operational efficiency but also ensure adherence to regulatory requirements. By focusing on comprehensive quality management systems, personnel training, facility design, and rigorous documentation practices, organizations can create a robust framework that supports compliance and fosters a culture of excellence.
Key insights discussed include:
- The importance of continuous education and the role of regular audits in identifying areas for improvement.
- The integration of advanced technologies in quality control and laboratory testing, which further underscores the necessity of staying current with evolving regulations.
Organizations that prioritize these strategies are better equipped to navigate the complexities of cGMP compliance, ultimately leading to improved product quality and safety.
As the landscape of pharmaceutical manufacturing continues to evolve, embracing these strategies becomes increasingly vital. Companies are encouraged to invest in their workforce, enhance their operational processes, and remain vigilant in their adherence to cGMP standards. By doing so, they not only protect their reputation but also contribute to the overarching goal of delivering safe and effective products to the market. This commitment ensures patient well-being and fosters trust in the healthcare system.
Frequently Asked Questions
What is the focus of AVS Life Sciences regarding cGMP principles?
AVS Life Sciences provides comprehensive guidance on cGMP medical practices specifically designed for regulatory officers, focusing on critical sectors such as APIs, pharmaceutical products, and testing facilities to ensure effective implementation of current cGMP principles.
How does AVS Life Sciences support compliance officers?
AVS Life Sciences helps compliance officers deepen their understanding of cGMP medical practices, facilitating their application within organizations to mitigate risks and enhance operational efficiency, thereby transforming GMP facilities and ensuring compliance for biotechnology clients.
Why is a Quality Management System (QMS) important for cGMP practices?
A QMS is essential for achieving effective cGMP practices as it provides a systematic approach to managing standards across operations, ensuring processes are standardized and comply with regulatory requirements, including GXP and FDA regulations.
What are the key components of an effective Quality Management System?
The key components include Quality Planning, Quality Control, Quality Assurance, Quality Improvement, Standard Operating Procedures (SOPs) Development, and addressing Data Integrity Deviations.
What benefits do organizations experience by employing effective QMS practices?
Organizations with effective QMS practices experience a 25% higher customer retention rate and a 40% reduction in customer complaints.
How does personnel training contribute to cGMP compliance?
Comprehensive training programs ensure that staff are qualified and knowledgeable about cGMP regulations, operational procedures, and standards, reducing the risk of non-compliance and enhancing product excellence.
What role do personnel qualifications play in cGMP practices?
Well-informed personnel are better equipped to navigate complex regulatory environments and uphold the integrity of production processes, which is crucial for maintaining compliance with cGMP standards.
Can you provide an example of the impact of effective training programs in a biotechnology facility?
AVS Life Sciences' enhancement of a biotechnology GMP facility demonstrated that effective training programs can lead to significant improvements in adherence rates and foster a culture of quality and safety.
What challenges can arise during the implementation of cGMP practices?
Challenges such as anomalies in test results due to improperly installed equipment can occur, highlighting the need for continuous evaluation and improvement of testing protocols and business processes.
