10 Key Insights for Navigating the CGMP Environment

Overview
The article presents critical insights for effectively navigating the Current Good Manufacturing Practices (CGMP) environment, particularly within the pharmaceutical and biotechnology sectors. It underscores the necessity of strict adherence to CGMP as a means to mitigate regulatory risks, enhance operational efficiency, and ensure product safety. Supported by statistics illustrating compliance challenges and successful case studies, the article demonstrates the tangible benefits of implementing robust CGMP strategies. By engaging with these insights, readers are encouraged to consider the importance of compliance solutions that can significantly impact their operational practices.
Introduction
The landscape of pharmaceutical manufacturing is becoming increasingly complex, with compliance with Current Good Manufacturing Practices (CGMP) more critical than ever. As regulatory scrutiny intensifies, organizations must not only grasp these standards but also skillfully navigate the challenges they present. This article explores ten key insights that illuminate the path to effective CGMP adherence, providing invaluable guidance for industry professionals aiming to enhance product safety and operational efficiency. How can organizations leverage these insights to not only meet regulatory demands but also secure a competitive edge in an evolving market?
AVS Life Sciences: Key Insights on Current Good Manufacturing Practices (CGMP)
AVS Life Sciences provides essential guidance on Current Good Manufacturing Standards in a cgmp environment, empowering clients in the pharmaceutical and biotechnology sectors to navigate regulatory complexities effectively. The significance of is underscored by the FDA's documentation of deficiencies in over 5,000 Form 483s, indicating widespread compliance challenges. In fiscal year 2021, the percentage of non-compliant samples escalated to 35%, a stark increase from 16% the prior year, underscoring the pressing need for robust compliance strategies.
Real-world examples demonstrate the successful application of [current good manufacturing practices](https://avslifesciences.com/global-capabilities). For instance, AVS Life Sciences assisted a leading biotechnology firm in upgrading its manufacturing area to align with current good manufacturing practices, completing the project on time and within budget. This case exemplifies how adherence to the CGMP environment not only mitigates regulatory risks but also enhances operational efficiency and product integrity.
Current trends indicate a growing emphasis on data integrity and management systems, particularly as the biologics market is projected to expand at a compound annual growth rate of 15% by 2025. The FDA's New Inspection Protocol Project (NIPP) aims to improve inspection efficacy through a targeted, data-driven approach, reflecting the industry's shift towards more sophisticated regulatory practices.
Professional insights highlight the necessity of a CGMP environment, with industry leaders advocating for a balanced strategy that emphasizes both efficiency and quality enhancement. Organizations that commit to good manufacturing practices not only safeguard public health but also bolster their market reputation, ultimately leading to greater success in a competitive landscape.
Understanding CGMP: Definition and Core Principles
Current Good Manufacturing Practices (CGMP) are regulations enforced by the FDA to ensure that manufacturing processes and facilities are designed, monitored, and controlled effectively. The core principles of CGMP encompass several critical aspects:
- Clean and Hygienic Environments: Maintaining a clean manufacturing environment is essential to prevent contamination and ensure product safety. Businesses that adhere to good manufacturing practices implement strict sanitation protocols and consistently educate staff on hygiene methods.
- Consistent Production and Quality Control: Current Good Manufacturing Practices mandate that products are consistently produced and controlled according to established quality standards. This includes conducting in-process controls and testing to verify that each batch meets predefined specifications.
- Robust Documentation Practices: Accurate and complete documentation is a cornerstone of current good manufacturing practices. It provides an audit trail for regulatory verification, ensuring that all processes—from raw material sourcing to final product release—are well-documented and traceable.
A significant example of successful compliance with good manufacturing practices is AVS Life Sciences' recent project, where they assisted a pharmaceutical manufacturer in enhancing their facility from a Biosafety Level 1 to a Level 2 GMP environment for the production of lentivirus vector material. This transformation involved a comprehensive gap analysis, equipment installation, and rigorous validation processes, ensuring and GXP standards. AVS Life Sciences' meticulous documentation practices throughout this project demonstrated full traceability, which was positively received by the client's quality assurance team.
Statistics indicate that failure to adhere to good manufacturing practices can result in major consequences, such as costly product recalls and FDA-issued warning letters. For instance, the FDA has not recognized any process models that validate foundational assumptions throughout the manufacturing process, underscoring the necessity for strict compliance with current good manufacturing practices.
Examples of companies successfully maintaining clean manufacturing environments under the CGMP environment include those that have adopted advanced manufacturing technologies and continuous improvement practices. These organizations not only comply with regulatory standards but also enhance operational efficiency and foster consumer trust through their dedication to excellence and safety.

Importance of CGMP: Ensuring Product Safety and Quality
The [cgmp environment](https://avslifesciences.com/industry-news/advancements-in-medical-research) is vital for ensuring that pharmaceutical products are not only safe and effective but also meet the highest standards. By rigorously adhering to a cgmp environment, organizations can significantly mitigate the risks of contamination, mix-ups, and manufacturing errors. This commitment not only protects consumer health but also enhances the credibility and reputation of the company in the competitive pharmaceutical landscape.
A transformative case study exemplifying this is AVS Life Sciences' successful upgrade of a biotechnology client's manufacturing facility from a Biosafety Level 1 GMP to a Level 2 GMP. This project encountered challenges, such as the oversight of barcode scanner camera installations, which initially resulted in anomalies in test results. However, the project was completed on schedule and within budget, demonstrating how effective assurance practices can bolster compliance and operational efficiency.
Companies that have embraced a cgmp environment, such as AVS Life Sciences, report improved operational efficiencies and heightened public trust, which contribute to a stronger market position. As the industry evolves in 2025, the influence of cGMP on product quality will become increasingly evident, with specific statistics indicating that organizations adhering to these practices experience fewer product recalls and legal challenges. Ultimately, the transcends mere regulatory compliance; it represents a strategic advantage that fosters consumer safety and fortifies the integrity of the pharmaceutical supply chain.
FDA Compliance Assessment: How the FDA Evaluates CGMP Adherence
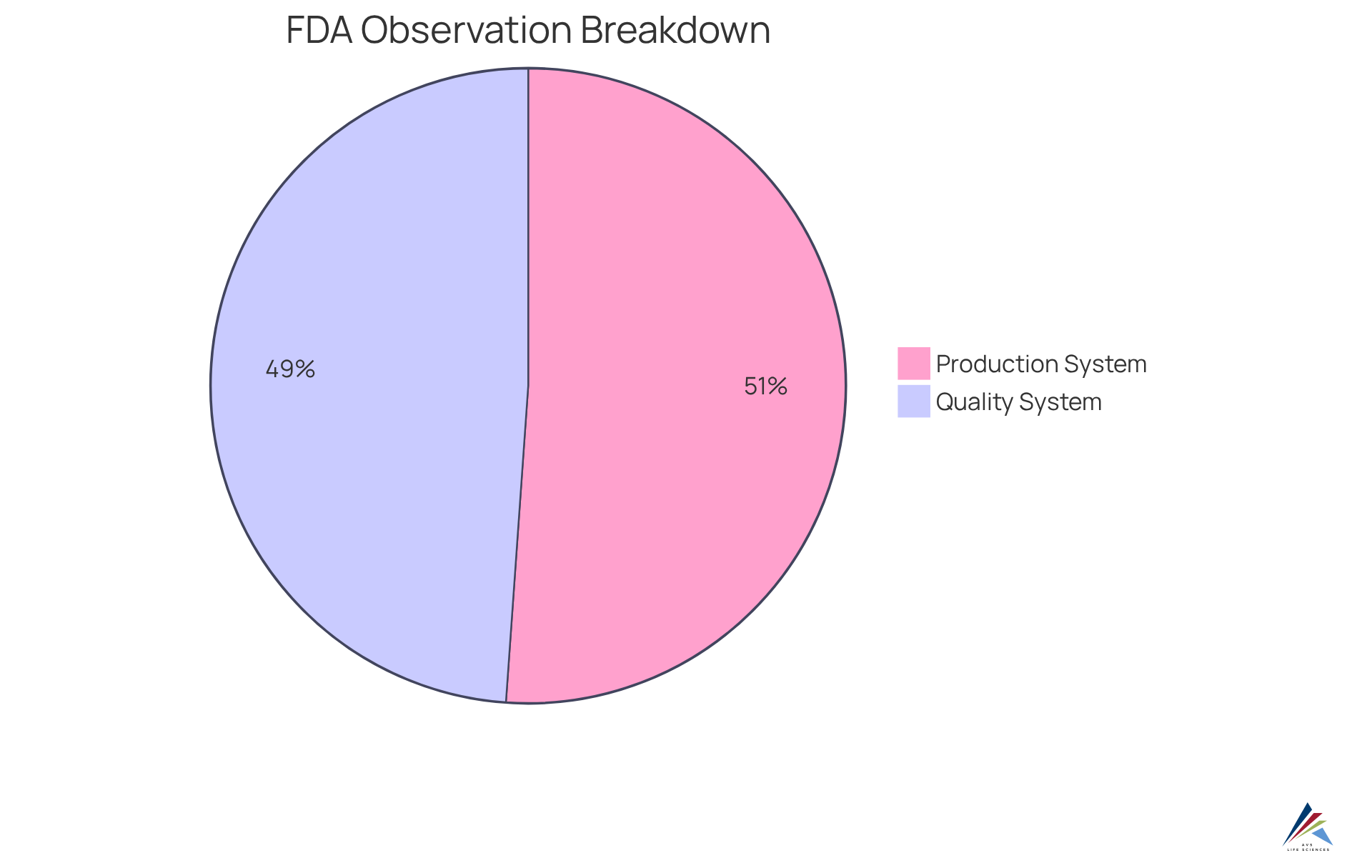
The FDA evaluates adherence to the CGMP environment through systematic inspections and audits of manufacturing facilities, focusing on documentation, processes, and conformity to established regulations. Organizations must be prepared to demonstrate their commitment to these standards within a CGMP environment, which involves maintaining detailed records that facilitate a smooth inspection process. Notably, statistics reveal that:
- The Production System accounted for 27.4% of total FDA 483 observations from 2018 to 2021.
- The Quality System closely followed with 26.2%.
This highlights during audits. Insights from regulatory specialists suggest that a proactive strategy for documentation and standards management in a CGMP environment can significantly enhance the likelihood of successfully passing inspections. Effective audits often depend on an organization’s ability to showcase efficient quality control practices in a CGMP environment and foster a culture of adherence. This can lead to favorable evaluations from FDA inspectors and a reduction in identified deficiencies.
Consequences of Non-Compliance: Risks to Drug Safety
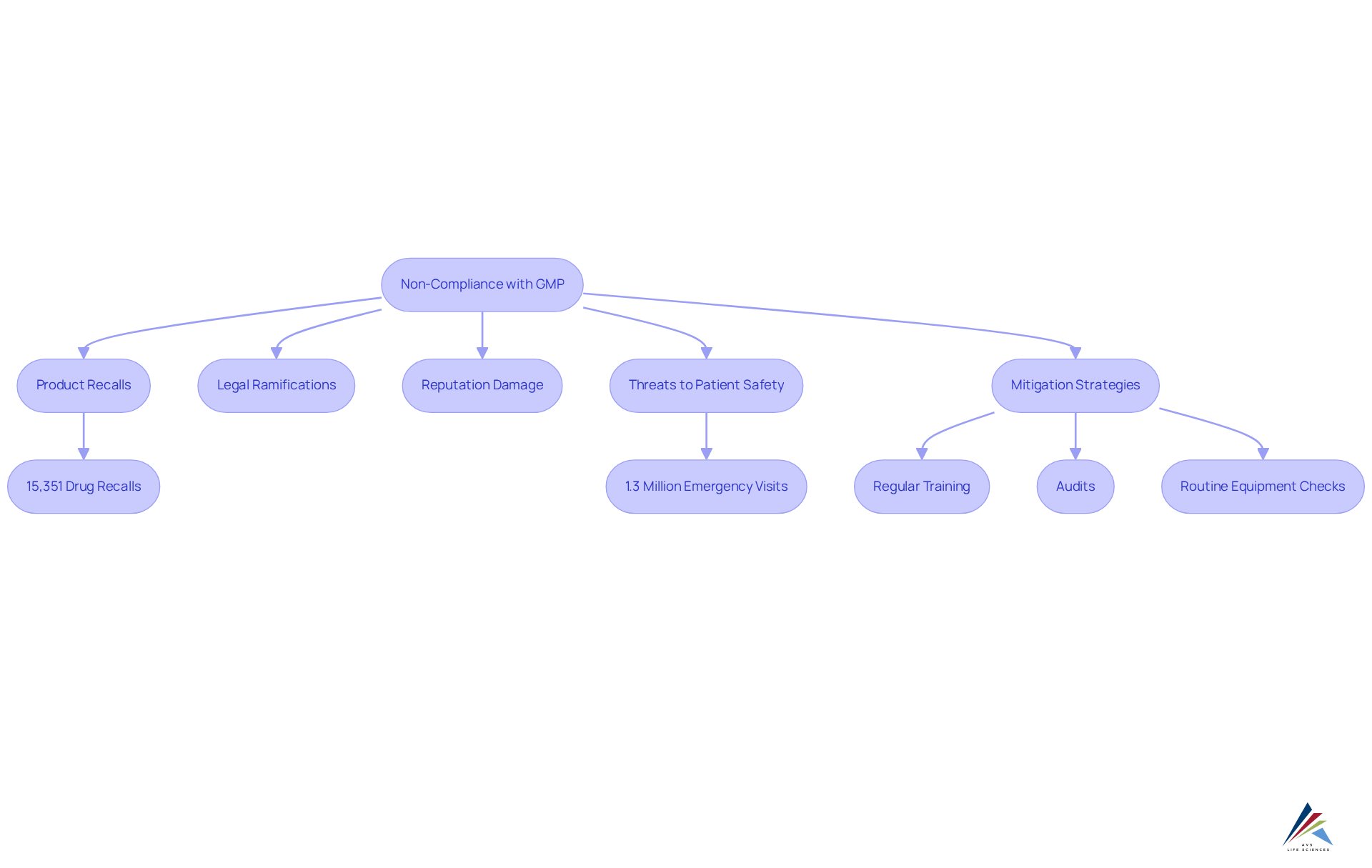
Non-compliance with Current Good Manufacturing Standards in a cgmp environment poses severe consequences, including product recalls, legal ramifications, and detrimental impacts on a company's reputation. The FDA has reported over 15,351 drug recalls in the U.S. since 2012, with 94% occurring nationally, underscoring the prevalence of regulatory failures. Such violations compromise the integrity of pharmaceutical products and significantly threaten patient safety, potentially leading to adverse health outcomes.
For example, adverse drug events result in approximately 1.3 million emergency department visits annually, highlighting the critical need for stringent adherence to the cgmp environment. Organizations must recognize that maintaining these standards in a cgmp environment transcends mere regulatory obligation; it is a moral imperative to protect public health and ensure product safety.
The fallout from violations within a cgmp environment extends beyond immediate legal and financial repercussions, fostering long-term trust deficits with consumers and stakeholders, ultimately jeopardizing business viability. A transformative case study from AVS Life Sciences exemplifies this: by assisting a leading biotechnology company in upgrading their manufacturing area from a Biosafety Level 1 GMP facility to a Level 2 GMP facility, AVS enabled the client to focus on developing life-saving medicines while upholding rigorous quality assurance standards.
During this transition, discrepancies were identified in test results due to improperly installed barcode scanner cameras, which had been initially overlooked. This experience prompted the QC laboratory and Quality teams to reassess their processes, resulting in enhanced testing protocols and improved team communication.
To mitigate risks, regulatory officers should implement regular training and audits, drawing lessons from like that of AVS Life Sciences. A practical recommendation for regulatory officers is to establish routine examinations of equipment and procedures to identify potential issues before they escalate into regulatory failures.
FDA Actions Against CGMP Violations: Protecting Public Health
The FDA wields significant authority in addressing violations within the cgmp environment, employing a range of measures such as issuing warning letters, imposing penalties, and even shutting down non-compliant production facilities. In FY23 alone, the agency issued 180 warning letters to drug and biologics manufacturers, indicating a marked increase in enforcement efforts as the FDA resumed regular inspection operations following the COVID-19 pandemic. These proactive measures are vital for , ensuring that only safe and effective products reach the market.
Notably, the FDA's emphasis on over-the-counter (OTC) products resulted in 37 warning letters targeting compliance issues in categories such as hand sanitizers and sunscreens, highlighting persistent safety concerns. Companies that fail to adhere to a cgmp environment face severe repercussions, as highlighted by recent cases where organizations were cited for significant deficiencies in quality control and data integrity.
The consequences of CGMP non-compliance in a cgmp environment extend beyond regulatory sanctions; they can threaten public health, as shown by instances where contaminated products infiltrated the market, posing serious risks to consumers. Therefore, organizations must remain vigilant in their compliance efforts to mitigate these risks and contribute to the overall safety of the pharmaceutical landscape.

Learning CGMP: Resources for New Drug Companies
New drug companies have access to a wealth of resources to deepen their understanding of the cgmp environment. Key among these are FDA guidance documents, which outline essential adherence standards and expectations. Interacting with these documents enables organizations to grasp the fundamental concepts of the cgmp environment, ensuring they can develop effective adherence strategies from the outset. Importantly, the FDA emphasizes the adaptability producers possess in determining 'significant phases' in the manufacturing process, which can aid in customizing regulatory strategies to specific operational requirements.
In addition to FDA resources, industry workshops and online training programs play a crucial role in educating teams about the cgmp environment requirements. Successful training programs often incorporate real-world case studies and interactive elements, enhancing both the learning experience and retention of critical information. For instance, programs that focus on practical applications within the cgmp environment have shown a significant improvement in adherence rates among participants. AVS Life Sciences offers tailored training solutions that empower organizations to implement these best practices effectively.
Expert opinions underscore that ongoing education and training are vital for ensuring adherence in a rapidly evolving regulatory landscape. The FDA advocates for a to in-process controls and testing, which is essential for establishing robust regulatory frameworks. By leveraging resources from AVS Life Sciences, new pharmaceutical firms can build a solid foundation within a cgmp environment, ultimately leading to improved product standards and regulatory success. Furthermore, manufacturers must substantiate their in-process controls scientifically, ensuring alignment with regulatory expectations.
Training Programs: Essential for CGMP Compliance
Implementing comprehensive training programs is essential for ensuring compliance with the cgmp environment throughout all levels of an organization. These programs must address key topics such as:
- Excellence control
- Documentation practices
- Regulatory requirements, including the critical stages of computer system validation (CSV)
Understanding the CSV process—planning, defining user requirements, design specifications, building and configuring systems, and conducting installation, operational, and performance qualifications—ensures that employees are well-versed in . Regular training sessions reinforce the significance of adherence and keep employees informed about any changes in regulations. This approach ultimately enhances quality control and strengthens the organization's commitment to regulatory standards.

Evolving CGMP Regulations: Staying Updated for Compliance
Good Manufacturing Practices regulations are continually evolving as the FDA updates its guidelines and industry standards advance. Organizations face that necessitate the implementation of robust processes for tracking regulatory updates and adjusting practices accordingly. Engaging with industry newsletters, attending relevant conferences, and joining professional organizations are effective strategies for staying abreast of the latest developments within the CGMP environment.
For instance, organizations that have adopted continuous process verification have reported a 23% decrease in waste and enhanced adherence rates. Furthermore, entities excelling in adherence often achieve 98-99% batch right-first-time rates, underscoring the importance of remaining knowledgeable. As regulatory scrutiny intensifies, proactive oversight of good manufacturing practices in a CGMP environment becomes not only advantageous but crucial for preserving operational integrity and maintaining a competitive edge.
Key Takeaways: Essential CGMP Facts for Compliance Officers
Oversight officers must prioritize the following essential good manufacturing practice takeaways:
- Good manufacturing practice is fundamental for ensuring product safety and quality in a cgmp environment, serving as a cornerstone of regulatory adherence.
- Non-adherence can result in significant repercussions, including financial penalties and damage to reputation.
- Continuous training and education are critical, with competency rates improving to 94% after practical verification.
- Staying informed about regulatory changes is vital, as 72% of pharmaceutical quality professionals report challenges in keeping pace with evolving requirements.
- Thorough documentation is indispensable for demonstrating adherence, as it helps prevent deviations from established protocols and supports organizational credibility. In fact, 40% of FDA warning letters cite data integrity violations, underscoring the necessity of meticulous record-keeping.
By adhering to these principles, compliance officers can adeptly navigate the complexities of the cgmp environment, ensuring not only compliance but also fostering a culture of quality and safety within their organizations.

Conclusion
Navigating the complexities of the Current Good Manufacturing Practices (CGMP) environment is crucial for organizations aiming to ensure product safety and quality in the pharmaceutical and biotechnology sectors. The insights shared highlight the importance of robust compliance strategies, as well as the evolving landscape of regulatory requirements that companies must adeptly manage to safeguard public health and maintain their market reputation.
Key arguments presented in the article underscore the necessity of adhering to CGMP standards, illustrated through real-world examples of successful compliance and the dire consequences of non-adherence. The emphasis on data integrity, comprehensive training programs, and continuous monitoring of regulatory updates are pivotal for organizations striving to achieve excellence in manufacturing practices. Moreover, the FDA's proactive measures against CGMP violations serve as a reminder of the critical nature of compliance in protecting consumers and ensuring the integrity of the pharmaceutical supply chain.
Ultimately, the commitment to a CGMP environment transcends regulatory obligation; it is a moral imperative that fosters consumer trust and enhances operational efficiency. Organizations are encouraged to prioritize ongoing education, embrace best practices, and remain vigilant in their compliance efforts. By doing so, they not only mitigate risks but also position themselves as leaders in a competitive landscape, ultimately contributing to the advancement of public health and safety.
Frequently Asked Questions
What are Current Good Manufacturing Practices (CGMP)?
Current Good Manufacturing Practices (CGMP) are regulations enforced by the FDA to ensure that manufacturing processes and facilities are designed, monitored, and controlled effectively to guarantee product safety and quality.
Why are CGMP important in the pharmaceutical industry?
CGMP are vital for ensuring that pharmaceutical products are safe, effective, and meet high standards, thereby mitigating risks of contamination, mix-ups, and manufacturing errors, which protects consumer health and enhances company credibility.
What are the core principles of CGMP?
The core principles of CGMP include maintaining clean and hygienic environments, ensuring consistent production and quality control, and implementing robust documentation practices for traceability and regulatory verification.
Can you provide an example of successful CGMP compliance?
AVS Life Sciences assisted a pharmaceutical manufacturer in enhancing their facility from a Biosafety Level 1 to a Level 2 GMP environment for lentivirus vector material production, demonstrating adherence to FDA regulations and GXP standards through meticulous documentation and validation processes.
What are the consequences of failing to adhere to CGMP?
Failure to comply with CGMP can lead to significant consequences, including costly product recalls and FDA-issued warning letters, highlighting the necessity for strict adherence to these practices.
How does the FDA monitor compliance with CGMP?
The FDA monitors compliance through inspections and documentation reviews, as indicated by the issuance of Form 483s that document deficiencies found during inspections.
What current trends are influencing CGMP practices?
There is a growing emphasis on data integrity and management systems, with initiatives like the FDA's New Inspection Protocol Project (NIPP) aiming to improve inspection efficacy through a targeted, data-driven approach.
How can organizations benefit from implementing CGMP?
Organizations that commit to CGMP can improve operational efficiency, enhance product integrity, safeguard public health, and bolster their market reputation, leading to greater success in a competitive landscape.
