10 Key Innovations Driving Life Sciences Digital Transformation

Overview
Key innovations driving life sciences digital transformation encompass:
- AI and machine learning
- Digital twins
- Cloud solutions
- Automation technologies
- Telemedicine
These advancements collectively enhance operational efficiency, compliance, and patient engagement. The article illustrates how these innovations not only streamline processes and improve data management but also ensure adherence to regulatory standards. Ultimately, they foster a more effective and responsive healthcare environment, addressing compliance challenges while promoting a proactive approach towards engagement with AVS Life Sciences.
Introduction
The life sciences sector is experiencing a significant transformation, propelled by a surge of innovative technologies that are set to redefine the delivery and management of healthcare. As organizations navigate intricate regulatory landscapes and pursue operational excellence, grasping the essential innovations at hand is crucial. This article explores ten pivotal advancements that not only enhance digital transformation but also tackle the pressing challenges of compliance and efficiency. How can these innovations be leveraged to meet regulatory demands while simultaneously elevating patient care in an increasingly competitive landscape?
AVS Life Sciences: Enhancing Digital Transformation with Quality Compliance Solutions
AVS Life Sciences plays a vital role in driving life sciences digital transformation within the pharmaceutical and biotechnology industries by offering customized quality assurance solutions. As organizations face intricate regulatory landscapes, these services empower them to embrace innovative technologies effectively.
By seamlessly integrating regulatory strategies with digital tools, AVS supports life sciences digital transformation, enabling clients to uphold rigorous quality standards throughout the product lifecycle. This commitment enhances operational efficiency and fosters a culture of continuous improvement, ultimately driving effectiveness in their operations.
Utilizing methodologies such as risk-based approaches and , AVS guarantees adherence and quality assurance. In an evolving industry, the integration of quality compliance solutions is increasingly vital for organizations aiming to thrive in a competitive landscape.
Engage with AVS Life Sciences to ensure your organization not only meets but exceeds compliance expectations.
AI and Machine Learning: Driving Innovation in Drug Discovery and Patient Care
Artificial Intelligence (AI) and machine learning are revolutionizing drug discovery and healthcare by significantly reducing the time and costs associated with bringing new therapies to market. These advanced technologies excel at analyzing extensive datasets, identifying potential drug candidates, and predicting patient responses with remarkable accuracy. For instance, AI-designed drugs boast an impressive 80-90% success rate in Phase I trials, compared to the traditional 40-65% success rate, underscoring their potential to enhance drug development efficiency.
As organizations increasingly adopt AI, ensuring compliance with legal standards becomes paramount. The life sciences digital transformation must prioritize safety and efficacy in the integration of AI, necessitating a robust compliance framework. This involves tackling challenges such as data variability and , which are crucial for securing regulatory approval. AVS Life Sciences highlights the significance of adhering to FDA regulations and GXP standards, including the management of Data Integrity Deviations and the establishment of Standard Operating Procedures (SOPs). Effectively implementing AI within these frameworks not only advances healthcare but also fosters trust in the technologies reshaping the healthcare landscape.
Furthermore, the application of AI can streamline clinical workflows, enhance safety, and facilitate personalized treatment strategies. By harnessing AI-driven insights, healthcare providers can make informed decisions that align with regulatory requirements, ultimately resulting in improved patient outcomes and more efficient drug development processes. As the industry evolves, collaboration among regulators, industry leaders, and ethical experts will be vital in navigating the complexities of AI applications within the context of life sciences digital transformation. Testimonials from clients, including those from MSAT Roche and Five Prime-SSF, illustrate the successful integration of AI in compliance-driven projects, showcasing the tangible benefits of these technologies in real-world applications.

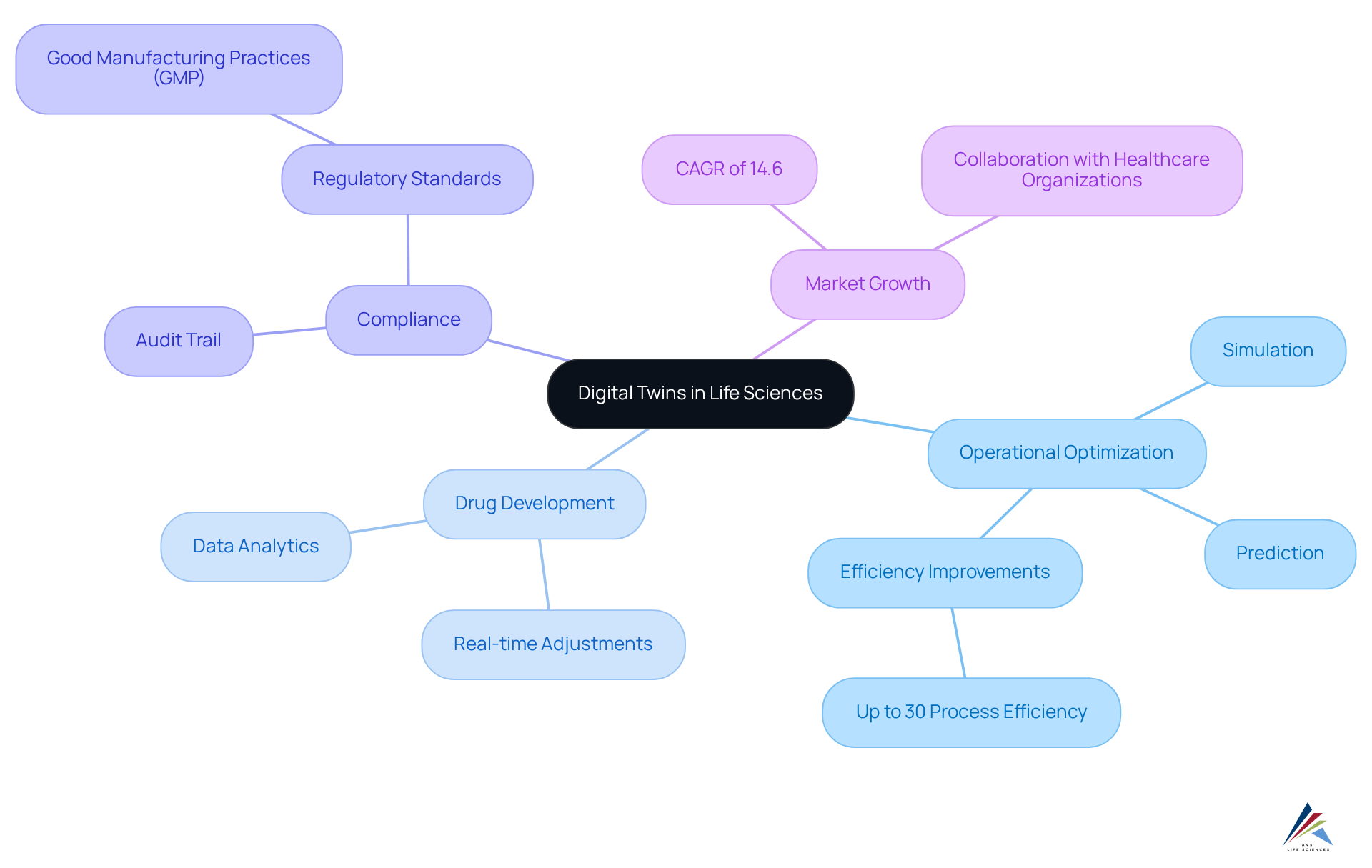
Digital Twins: Optimizing Operations and Product Development in Life Sciences
Digital twins serve as virtual replicas of physical systems, empowering organizations to simulate, predict, and optimize their operations effectively. In the life sciences digital transformation sector, these digital models play a crucial role in modeling drug development processes, allowing for real-time adjustments driven by data analytics. This innovation markedly boosts operational efficiency; studies reveal that organizations leveraging digital twins can achieve process efficiency improvements of up to 30%.
Furthermore, digital twins bolster compliance with legal standards by creating a transparent audit trail of modifications and decisions throughout the development process. This feature is vital for ensuring adherence to (GMP) and other regulatory standards, ultimately streamlining regulatory submissions and approvals.
AVS Life Sciences exemplifies the successful integration of digital twins in their transformative facility upgrades, notably facilitating the transition of a leading biotechnology manufacturer from a Biosafety Level 1 to a Level 2 GMP facility. Their comprehensive approach to computer system validation, which includes meticulous stages, testing, and regulatory compliance, ensures that all operational changes are thoroughly documented and aligned with industry standards.
As the market for digital twins is projected to grow at a compound annual growth rate (CAGR) of 14.6%, their role in life sciences digital transformation, particularly in enhancing drug development processes and ensuring compliance, is becoming increasingly indispensable for organizations striving to navigate the complexities of the industry effectively.
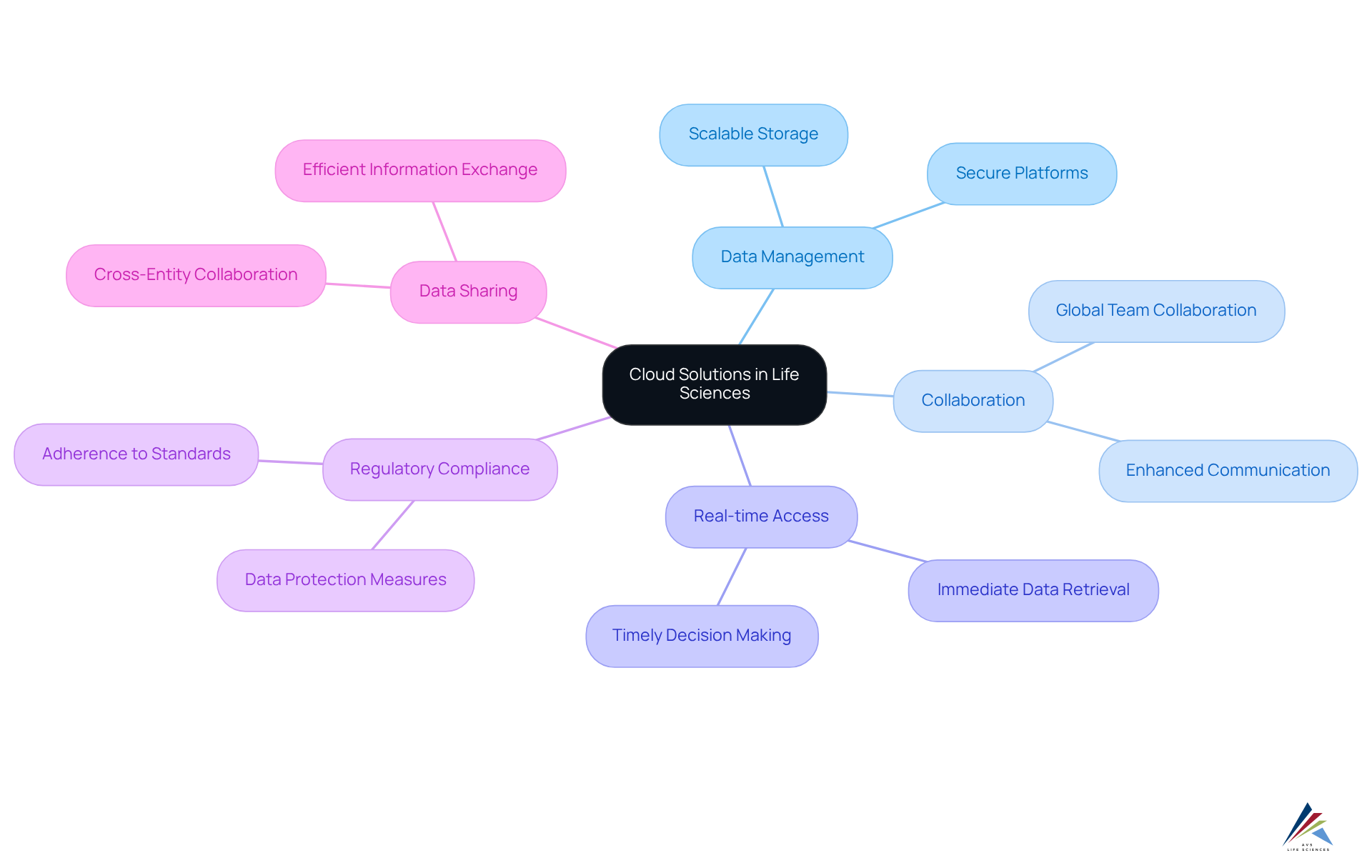
Cloud Solutions: Facilitating Data Management and Collaboration in Life Sciences
Cloud solutions have revolutionized data management within the life sciences digital transformation by providing scalable and secure platforms for the storage and sharing of vital information. These advanced technologies not only facilitate but also enable real-time access to critical data. Furthermore, cloud solutions can be meticulously designed to comply with regulatory standards, ensuring that sensitive information is effectively safeguarded while promoting efficient data sharing and analysis across various entities.
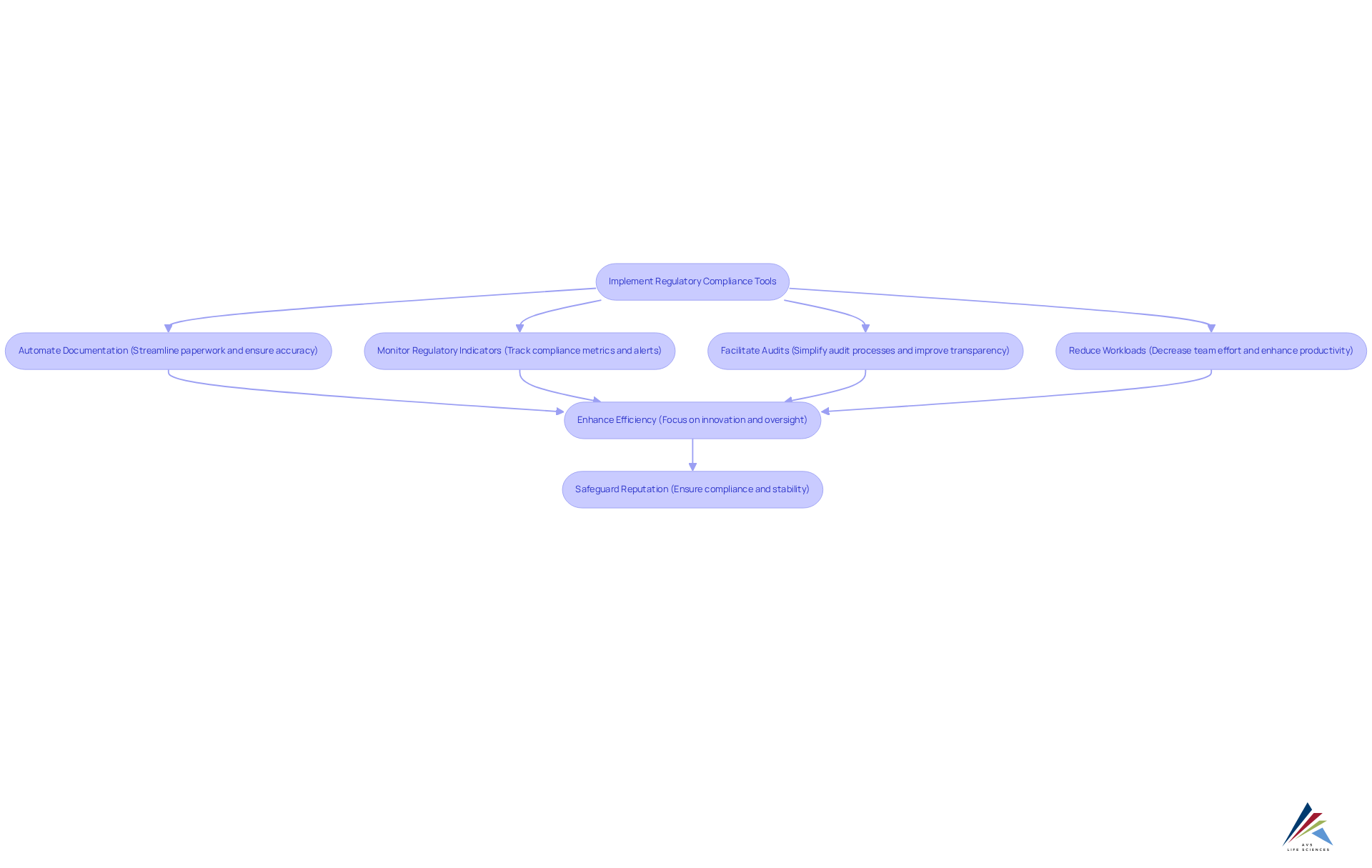
Regulatory Compliance Tools: Streamlining Processes for Life Sciences Organizations
Regulatory adherence tools are essential for life sciences companies facing compliance challenges, enabling them to streamline processes and align with industry standards. These advanced solutions not only automate documentation and monitor regulatory indicators but also facilitate audits, significantly reducing team workloads and minimizing the risk of rule violations. By leveraging automation in regulatory adherence, organizations can enhance efficiency and focus on innovation while ensuring the necessary oversight within the life sciences digital transformation. This proactive approach and contributes to its long-term success and stability. Industry leaders recognize that understanding and implementing these compliance tools is crucial for effectively managing risks and maintaining ethical and legal business practices.
Patient Engagement Platforms: Revolutionizing Interactions in Life Sciences
Engagement platforms are fundamentally transforming the dynamics between healthcare providers and individuals, streamlining communication, education, and support. These innovative technologies empower individuals to actively engage in their healthcare journeys, significantly enhancing adherence to treatment protocols. In fact, involved individuals are 30% less likely to be readmitted to the hospital after discharge, underscoring the importance of effective communication and collaboration in achieving improved health outcomes.
By harnessing technology as part of life sciences digital transformation to enhance user engagement, organizations can ensure that their products are utilized effectively and safely, aligning with stringent regulatory requirements. The shift towards a more participatory model not only improves client satisfaction but also elevates the overall quality of care. As healthcare organizations increasingly adopt tools like mobile applications and portals for individuals, they become better equipped to meet the evolving demands of a tech-savvy community.
Moreover, the incorporation of real-time data insights enables providers to identify and address gaps in care delivery, thereby enhancing the experience of individuals. This approach cultivates trust and results in , making individual involvement a crucial component of contemporary healthcare strategies. As industry specialists note, the most groundbreaking research occurs at the intersection of scientific knowledge and personal experience, highlighting the critical role of individual participation in developing effective healthcare solutions.

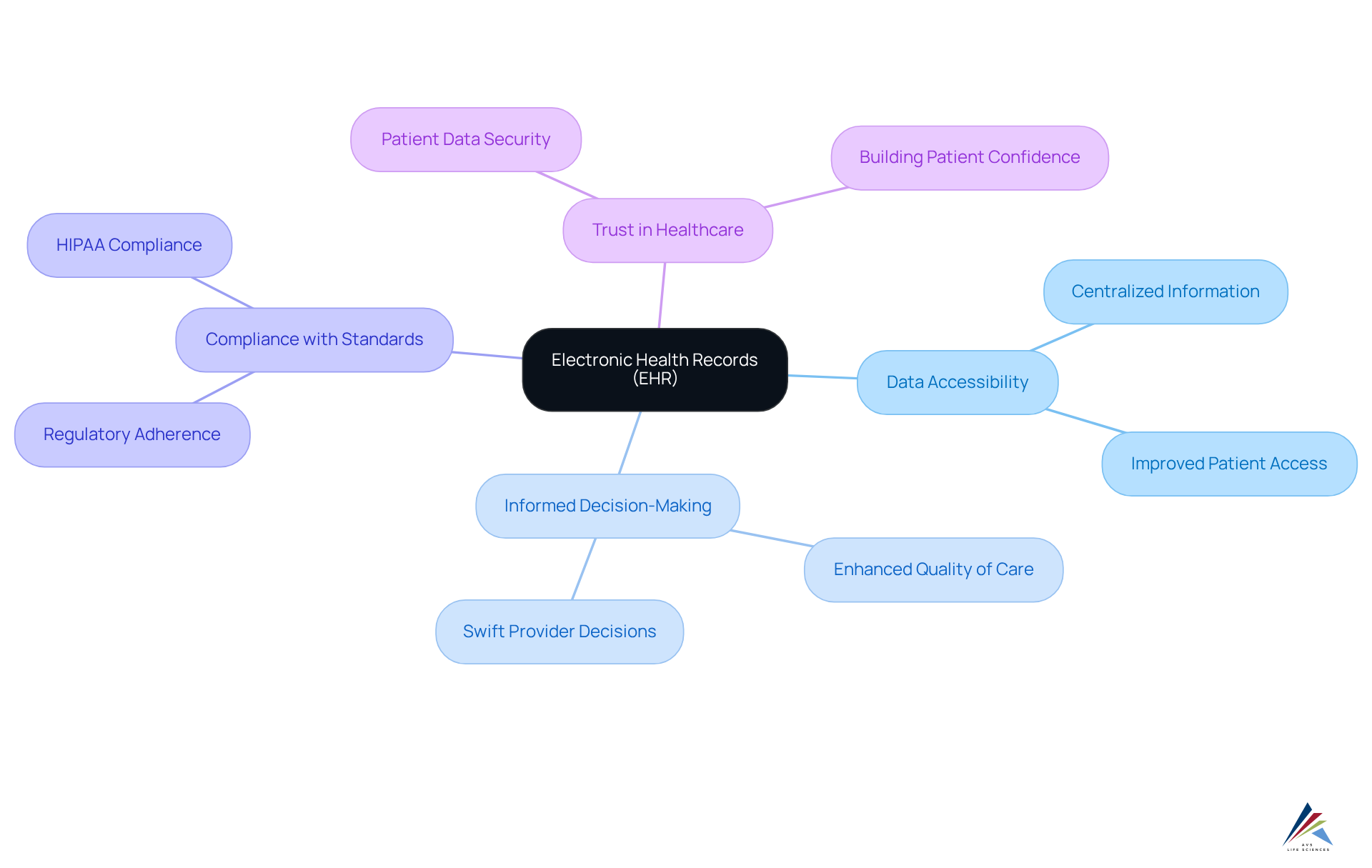
Electronic Health Records (EHR): Improving Data Accessibility and Patient Care
Electronic Health Records (EHR) systems play a pivotal role in enhancing data accessibility and ensuring continuity of care for individuals. By centralizing comprehensive individual information, EHRs enable healthcare providers to make swift, informed decisions, thereby significantly improving the quality of care delivered.
Compliance with official standards is not merely a regulatory requirement; it is essential for safeguarding the security and privacy of patient data. This adherence not only fulfills legal obligations but also .
Consequently, EHRs emerge as an indispensable asset in the life sciences digital transformation landscape, highlighting their critical importance in the pursuit of effective healthcare solutions.
Automation Technologies: Boosting Efficiency and Accuracy in Life Sciences
The life sciences digital transformation is being driven by automation technologies that are revolutionizing the sector, particularly in pharmaceuticals, significantly enhancing efficiency and accuracy across various processes. Laboratory automation, for instance, minimizes human error and accelerates workflows, allowing researchers to focus on more complex tasks. Automated data entry systems streamline information management, reducing the time spent on manual processes and increasing overall productivity.
The advantages of adopting automation extend beyond simple efficiency improvements; they fulfill a crucial role in ensuring adherence to stringent industry standards. By automating routine tasks, companies can maintain high levels of accuracy in documentation and reporting, which is essential for meeting regulatory requirements. This alignment with regulations not only mitigates risks but also fosters a culture of quality within the organization.
Successful examples of automation enhancing efficiency in pharmaceutical processes are evident in various case studies. For instance, AI-driven solutions have been implemented to optimize drug discovery, shortening timelines by one to two years and substantially reducing costs associated with the development phase. Moreover, organizations that have embraced automation report improved employee engagement, as automation alleviates the burden of repetitive tasks, allowing staff to engage in more significant work.
As the sector experiences life sciences digital transformation, the integration of automation technologies is expected to drive further advancements in operational efficiency and compliance, ultimately leading to improved outcomes in drug development and patient care. This not only enhances productivity but also positions organizations to thrive in an increasingly competitive landscape.
Digital Clinical Trials: Transforming Research Methodologies in Life Sciences
Digital clinical trials are revolutionizing research methodologies within the life sciences, harnessing advanced technology to significantly enhance participant engagement and streamline data collection. By employing tools such as remote monitoring, telehealth, and mobile applications, these trials not only improve the efficiency of the research process but also increase accessibility for participants.
However, as organizations embrace this digital transformation, it is crucial to maintain strict adherence to regulatory requirements. This commitment ensures the and safeguards participant safety, ultimately fostering trust in the findings.
The successful implementation of digital trials hinges on a careful balance between innovation and compliance with established guidelines, making it essential for life sciences companies to integrate robust compliance strategies into their digital frameworks. As the landscape of clinical research evolves, organizations must prioritize compliance to navigate the complexities of regulatory environments effectively.
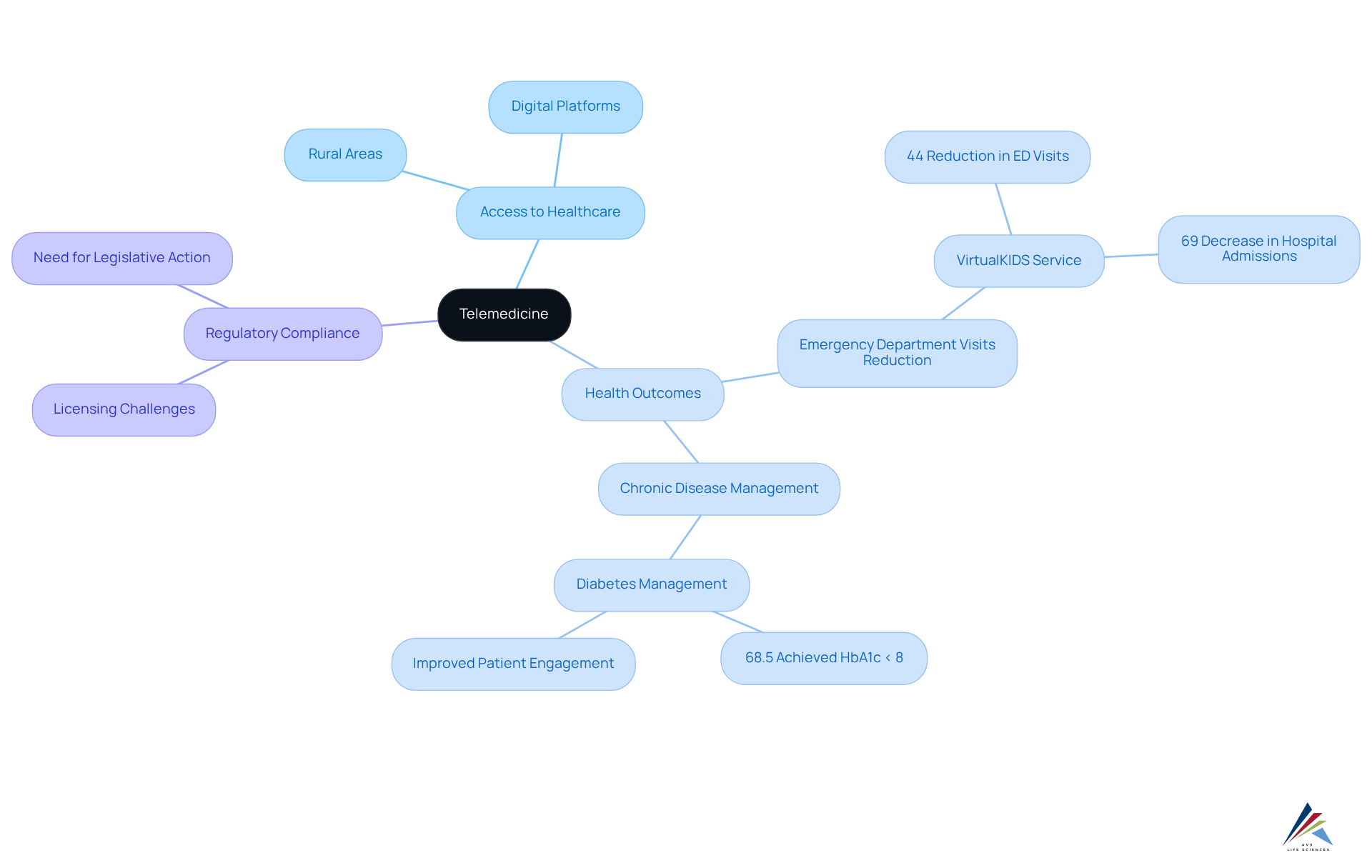
Telemedicine: Expanding Patient Access and Supporting Digital Transformation
Telemedicine has emerged as a crucial innovation in broadening access to healthcare services, particularly in rural and underserved areas. By leveraging digital platforms for consultations and follow-ups, telemedicine significantly enhances convenience and engagement for individuals. A notable example is the nursing-led telehealth service, VirtualKIDS, which achieved a remarkable 44% reduction in emergency department visits and a 69% decrease in hospital admissions. This demonstrates the effectiveness of remote care in managing non-urgent cases.
As telemedicine continues to advance, organizations must prioritize adherence to regulatory standards to protect individual privacy and ensure quality care. Current regulations often require providers to obtain a license in the individual's state, complicating access for those seeking care across state lines. This underscores the to create a more flexible framework that accommodates modern healthcare delivery methods.
Moreover, studies indicate that telemedicine interventions have led to significant improvements in individual health outcomes, particularly in chronic disease management. Telemedicine has been linked to a significant decrease in HbA1c levels among individuals with diabetes, with 68.5% reaching levels below 8% through video consultations. Such outcomes underscore the potential of telemedicine to not only enhance access but also improve the overall quality of care delivered.
In summary, as telemedicine reshapes the healthcare landscape, its integration into routine practice must be accompanied by a commitment to regulatory compliance and quality assurance. This ensures that patients receive the best possible care through these innovative solutions.
Conclusion
The landscape of life sciences is undergoing a profound transformation driven by innovative technologies that enhance efficiency, compliance, and patient engagement. This article highlights how advancements such as AI, digital twins, cloud solutions, and telemedicine are not only optimizing operations but also ensuring that organizations can navigate the complex regulatory environment effectively. This evolution is essential for meeting the increasing demands of the industry and improving healthcare outcomes.
Key insights discussed include:
- The significant role of AI and machine learning in drug discovery, which have demonstrated a remarkable increase in success rates during clinical trials.
- The use of digital twins and cloud solutions has proven to enhance operational efficiency and facilitate collaboration among global teams.
- Regulatory compliance tools and patient engagement platforms have emerged as critical elements in ensuring adherence to industry standards while improving the overall quality of care.
As organizations continue to embrace these technologies, the importance of maintaining a strong compliance framework cannot be overstated. By prioritizing regulatory adherence and leveraging innovative solutions, life sciences companies can not only improve their operational effectiveness but also foster trust and satisfaction among patients. The ongoing digital transformation in life sciences is not merely a trend; it represents a pivotal shift towards a more efficient, patient-centric healthcare system that is equipped to meet the challenges of the future.
Frequently Asked Questions
What role does AVS Life Sciences play in digital transformation within the pharmaceutical and biotechnology industries?
AVS Life Sciences provides customized quality assurance solutions that help organizations navigate complex regulatory landscapes, enabling them to effectively embrace innovative technologies and uphold rigorous quality standards throughout the product lifecycle.
How does AVS Life Sciences enhance operational efficiency?
By integrating regulatory strategies with digital tools and utilizing methodologies such as risk-based approaches and data analytics, AVS Life Sciences enhances operational efficiency and fosters a culture of continuous improvement.
What impact do AI and machine learning have on drug discovery and patient care?
AI and machine learning significantly reduce the time and costs associated with bringing new therapies to market, excel at analyzing extensive datasets, and improve the success rates of drug candidates in clinical trials.
What are the compliance challenges associated with the integration of AI in life sciences?
Compliance challenges include ensuring safety and efficacy, managing data variability, and achieving algorithmic transparency, all of which are crucial for securing regulatory approval.
How can AI improve patient outcomes and drug development processes?
AI can streamline clinical workflows, enhance safety, facilitate personalized treatment strategies, and provide healthcare providers with insights that align with regulatory requirements, leading to improved patient outcomes.
What are digital twins and how do they benefit life sciences organizations?
Digital twins are virtual replicas of physical systems that allow organizations to simulate, predict, and optimize operations. They enhance operational efficiency, improve compliance with legal standards, and create transparent audit trails throughout the development process.
What is the significance of compliance in the use of digital twins?
Digital twins help ensure compliance with Good Manufacturing Practices (GMP) and other regulatory standards by documenting modifications and decisions, which streamlines regulatory submissions and approvals.
Can you provide an example of how AVS Life Sciences has successfully integrated digital twins?
AVS Life Sciences facilitated the transition of a leading biotechnology manufacturer from a Biosafety Level 1 to a Level 2 GMP facility by integrating digital twins in their transformative facility upgrades, ensuring thorough documentation and regulatory compliance.
What is the market outlook for digital twins in life sciences?
The market for digital twins is projected to grow at a compound annual growth rate (CAGR) of 14.6%, highlighting their increasing importance in enhancing drug development processes and ensuring compliance within the life sciences sector.
