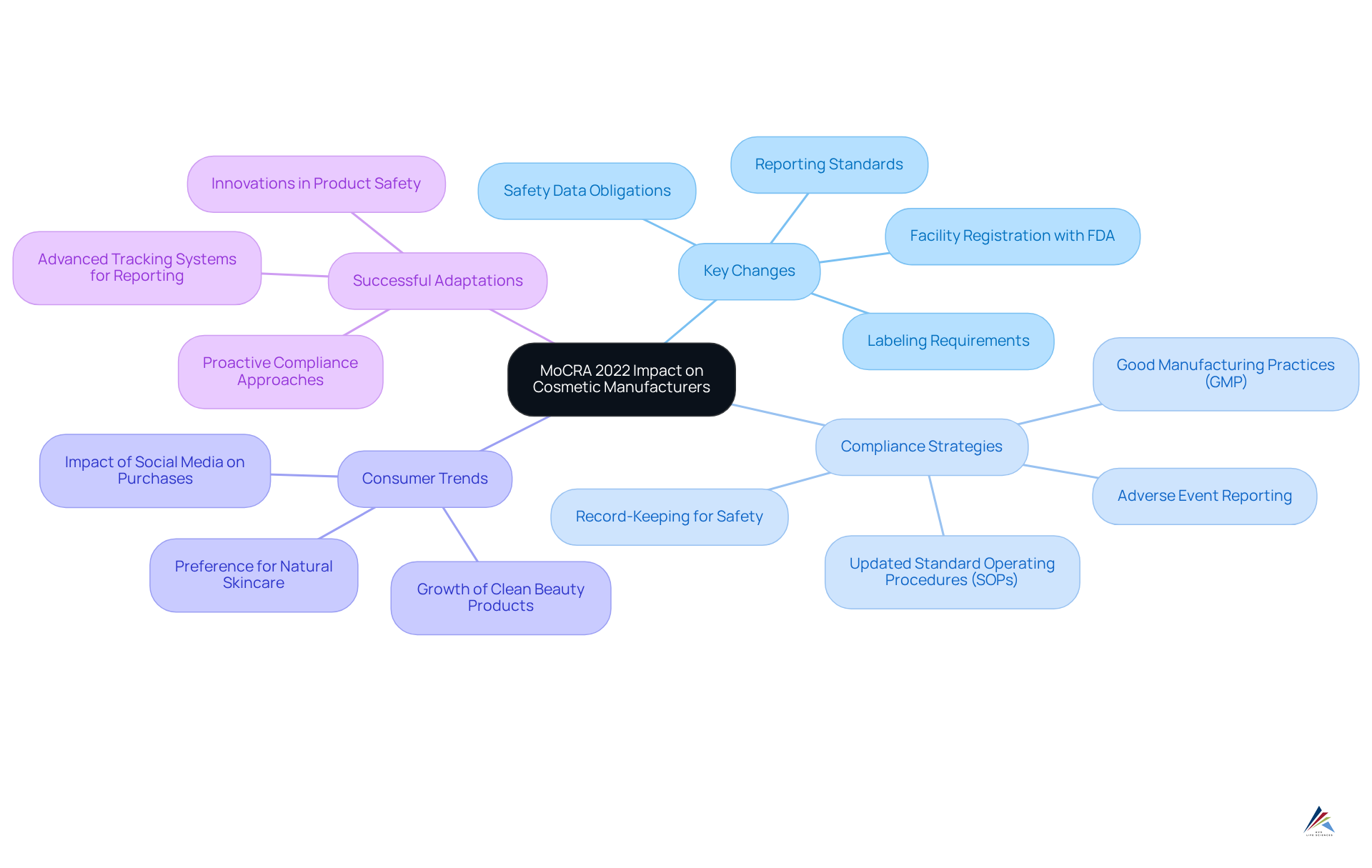
10 Key Changes Under MoCRA 2022 Explained for Cosmetic Manufacturers

Overview
The article highlights the significant changes introduced by the Modernization of Cosmetics Regulation Act (MoCRA) 2022 that impact cosmetic manufacturers. Key aspects include:
- New labeling requirements
- Enhanced safety data obligations
- Rigorous reporting standards
Manufacturers must adapt to these changes to ensure compliance, safeguard consumer trust, and mitigate legal risks. As the FDA's oversight has intensified, adherence to these regulations is essential for success in the evolving cosmetics market. By embracing these compliance solutions, manufacturers can navigate the complexities of the regulatory landscape and position themselves advantageously within the industry.
Introduction
The landscape of cosmetic manufacturing is experiencing a significant transformation with the introduction of the Modernization of Cosmetics Regulation Act of 2022 (MoCRA 2022). This pivotal legislation redefines compliance standards, emphasizing safety, transparency, and consumer trust in an industry that thrives on innovation and quality. As manufacturers face new labeling requirements, enhanced safety data obligations, and rigorous reporting standards, the stakes have never been higher.
The question arises: how can cosmetic producers effectively navigate this complex regulatory environment while ensuring they meet both consumer expectations and legal mandates? This article will explore essential strategies and solutions for compliance, guiding you towards successful adaptation in this evolving landscape.
AVS Life Sciences: Regulatory Compliance Solutions for MoCRA 2022
AVS Life Sciences stands at the forefront of specialized regulatory adherence solutions, expertly guiding manufacturers through the complexities as : key changes affecting cosmetic manufacturers. By prioritizing and , designed to help clients with efficiency and precision. Their team of seasoned experts, well-versed in the intricacies of the topic as outlined in MoCRA 2022 explained: key changes affecting cosmetic manufacturers, delivers actionable insights and strategies that not only align with the latest regulatory expectations but also empower clients to achieve compliance with confidence. This commitment to excellence positions AVS Life Sciences as a , ready to tackle the challenges of today’s regulatory environment.
Updated Labeling Requirements: Key Changes Under MoCRA 2022
: key changes affecting cosmetic manufacturers include significant updates to for beauty items, presenting that these manufacturers must navigate. Manufacturers are now required to ensure that labels include , specific usage instructions, and any necessary warnings. Furthermore, the regulations mandate that labels reflect any changes in formulation or safety data.
Compliance with the updated labeling requirements, as detailed in MOCRA 2022 explained: key changes affecting cosmetic manufacturers, is essential to avoid penalties and ensure . As highlighted by Crystal Maira, a Senior Consultant, 'MoCRA defines Responsible Person as the manufacturer, packer, or distributor of a beauty item whose name is displayed on the label.'
By December 29, 2024, all beauty item labels must also include the to facilitate consumer inquiries and reporting of adverse events. With customers utilizing an average of 6 to 12 beauty items daily, the significance of clear and precise labeling cannot be overstated.
Failure to comply with the , as detailed in MOCRA 2022 explained: key changes affecting cosmetic manufacturers, may result in , highlighting the importance of . Aligning with these new regulations is vital for and safeguarding public health.
Enhanced Safety Data Requirements: Implications for Cosmetic Products
MoCRA 2022 explained: key changes affecting cosmetic manufacturers highlight that manufacturers of beauty items face significant that require enhanced for their offerings. This includes detailed insights into the safety of ingredients, potential risks, and the results of safety assessments.
To navigate these requirements effectively, manufacturers must implement robust and maintain to demonstrate compliance. Non-compliance can lead to serious repercussions, including recalls and legal consequences, as the FDA now possesses over beauty items.
Statistics reveal that a notable percentage of recalls in the cosmetics sector arise from , underscoring the critical need for adherence to these new regulations. The deadline for submitting registration and listing information is July 1, heightening the urgency for compliance.
Furthermore, Laura Rich, Counsel, remarked, "I think the FDA is going to be a bit surprised as to how much information they are going to be getting in July," highlighting the necessity for .
The implications of MoCRA 2022 explained: key changes affecting cosmetic manufacturers extend beyond mere compliance; they necessitate a towards prioritizing safety and transparency in product development. As the , producers must proactively audit their , along with their contracted producers and ingredient suppliers, to mitigate risks and ensure consumer trust.
Adverse Event Reporting: New Obligations for Cosmetic Manufacturers
The (MoCRA 2022) explained: key changes affecting cosmetic manufacturers imposes critical new responsibilities on cosmetic producers regarding . Under this regulation, producers must report any associated with their products to the FDA within a specified timeframe. This shift underscores the necessity for producers to implement robust that can effectively track consumer feedback and safety incidents. Such systems are vital not only for compliance but also for risk mitigation, enabling producers to respond swiftly to potential safety concerns.
Statistics reveal that in 2025, the cosmetics sector recorded a significant number of , with over 1,000 incidents reported. This data highlights the urgent need for producers to enhance their monitoring capabilities. The FDA's updated requirements necessitate that these events be documented and reported promptly, reinforcing the importance of transparency and accountability in . By establishing efficient monitoring systems—such as and —manufacturers can better safeguard consumer health and ensure .
Moreover, the implications of MoCRA 2022 explained: key changes affecting cosmetic manufacturers extend to hemp and CBD beauty products, necessitating related to these items. This comprehensive approach to safety and compliance is crucial for navigating the complexities of the contemporary regulatory landscape.

New Regulatory Framework: Transforming Cosmetic Product Oversight
The MoCRA 2022 explained: marks a pivotal transformation in the regulatory landscape for cosmetics, significantly bolstering the . This legislation mandates that manufacturers comply with stricter guidelines, including and comprehensive safety substantiation for their products. As of January 1, 2025, there are 9,528 unique active , reflecting the industry's adaptation to these new requirements. The FDA now wields the authority to suspend facility registrations linked to , underscoring the critical importance of .
Manufacturers are proactively responding to these changes. For instance, many are revising their manufacturing processes and implementing robust to ensure adherence to the new standards. The beauty industry, valued at $49 billion, is increasingly focused on enhancing , as MoCRA 2022 explained: key changes affecting cosmetic manufacturers necessitate compliance with stringent regulations. Industry experts observe that this shift not only elevates safety standards but also fosters greater consumer trust in beauty products.
The FDA's expanded role under MoCRA encompasses , obligating responsible parties to report incidents to the FDA within 15 business days. This new requirement highlights the within the beauty industry. As companies navigate these changes, they must establish clear processes for compliance, including meticulous record-keeping and adverse event reporting, to mitigate legal and financial risks associated with non-compliance.
Exemptions Under MoCRA: What Manufacturers Should Know
The specifics of : include specific exemptions that must be recognized. Certain items, particularly those classified as low-risk or meeting defined criteria, may not be subject to the full spectrum of regulatory obligations.
It is imperative for manufacturers to conduct a . Engaging with is essential to ensure they are while considering MOCRA 2022 explained: and upholding overall .
Good Manufacturing Practices (GMP): Compliance Essentials Post-MoCRA
The MoCRA 2022 explained: key changes affecting cosmetic manufacturers highlight the essentiality of adherence to for cosmetic producers. GMP guarantees that products are consistently produced and controlled in accordance with established quality standards. To comply with these requirements, manufacturers must:
- Implement
- Provide ongoing training for their staff
This dedication to quality not only ensures but also significantly enhances . Statistics suggest that companies with strong experience enhanced quality, resulting in greater customer satisfaction and loyalty. For example, , with more than 300 skilled associates worldwide, demonstrates how a strong emphasis on GMP can improve results.
As the industry evolves, the importance of maintaining stringent GMP standards cannot be overstated, especially as MoCRA 2022 explained: key changes affecting cosmetic manufacturers have established a new regulatory landscape. As noted by quality management professionals, "A nickel ain’t worth a dime anymore," emphasizing the need for continuous improvement in quality practices.
Furthermore, it is essential to recognize the exclusions under MoCRA, which do not pertain to items aimed at changing appearance for over 24 hours without consumer removal, items that are injected, or those intended for internal use. This context is essential for manufacturers to navigate regulations effectively.
International Trade Implications: Navigating MoCRA's Global Impact
The implications of explained: are substantial for international trade in cosmetic products. Producers aiming to export must of dual regulations, as MoCRA 2022 explained: key changes affecting cosmetic manufacturers require adherence to both its standards and the specific requirements of the destination country. This dual adherence presents considerable challenges, as and adjust their practices accordingly.
Statistics indicate that a significant proportion of beauty product manufacturers face regulatory hurdles when exporting, underscoring the necessity for proactive measures. For instance, the FDA can access records related to cosmetic items under FDCA §610 if there is a reasonable belief of adulteration, highlighting the of .
Moreover, all items currently supplied must be recorded by December 29, 2023, emphasizing the urgency for compliance. Engaging with , such as those at , is essential for facilitating a smoother navigation through these , ensuring that products meet all legal requirements while preserving market access.
Training and Education: Preparing Compliance Officers for MoCRA
To successfully implement the : key changes affecting cosmetic manufacturers, manufacturers must prioritize . This requires a comprehensive understanding of the new requirements, , and robust strategies for overseeing adherence. Regular training sessions and workshops are essential to equip teams with the knowledge and skills necessary to navigate the evolving regulatory landscape, especially as : key changes affecting cosmetic manufacturers are implemented.
Best practices for educating teams on MoCRA 2022 explained: key changes affecting cosmetic manufacturers include utilizing diverse training modalities such as e-learning, in-person workshops, and hands-on simulations. Organizations can adopt a blended learning strategy that merges theoretical knowledge with practical applications, ensuring that regulatory officers can effectively apply what they learn in real-world scenarios.
Effective , which include MoCRA 2022 explained: key changes affecting cosmetic manufacturers, often incorporate case studies and interactive elements, allowing participants to engage actively with the material. These programs not only address the regulatory requirements but also emphasize the importance of within the organization.
, as industry professionals emphasize. As Fizza Nabeel, Content Head at DRIDER, stated, 'Measuring the effectiveness of training on regulations is not a luxury; it’s a necessity for businesses operating in a regulated environment.' Incorporating advanced metrics such as behavioral transformation and knowledge retention can significantly enhance the assessment of training effectiveness. Mock audits can also serve as a valuable tool to assess employees' readiness for real audits, further strengthening training programs.
By encouraging a culture of ongoing enhancement and information exchange, producers can improve their adherence capabilities and mitigate risks associated with non-adherence.
Overall Impact of MoCRA 2022: A Comprehensive Overview for Manufacturers
MoCRA 2022 explained: key changes affecting cosmetic manufacturers significantly alters the regulatory landscape for cosmetic producers, underscoring the critical necessity for compliance. Key changes encompass new labeling requirements, enhanced safety data obligations, and rigorous reporting standards. Producers are now mandated to register their facilities with the FDA and submit comprehensive listings, including detailed ingredient disclosures. This transformation not only aligns U.S. regulations with international safety standards but also cultivates greater in compliant brands.
To navigate these changes effectively, manufacturers must adopt robust . This entails updating Standard Operating Procedures (SOPs) to conform with and ensuring meticulous documentation of safety substantiation. Companies are required to retain records supporting product safety for six years, which is vital for defending against potential litigation.
Statistics indicate that a substantial portion of the industry is adapting to these regulatory requirements. For example, as of 2025, 43% of millennials and Gen Z favor natural skincare compared to 31% of the general U.S. consumer population, illustrating a consumer trend that shapes compliance strategies. This proactive approach not only mitigates legal risks but also enhances product safety, ultimately bolstering consumer confidence.
Noteworthy instances of producers successfully adjusting to MoCRA 2022 explained: key changes affecting cosmetic manufacturers include those who have implemented advanced tracking systems for , ensuring that serious incidents are reported to the FDA within the mandated 15 business days. Such adaptations are crucial in a landscape where the FDA's authority to suspend s for safety violations is increasingly pronounced.
In conclusion, the MoCRA 2022 explained: key changes affecting cosmetic manufacturers reshapes the regulatory framework for beauty products, compelling manufacturers to prioritize . By embracing these changes, companies can fulfill regulatory obligations and position themselves as leaders in safety and consumer trust within the evolving cosmetics market.
Conclusion
The Modernization of Cosmetics Regulation Act of 2022 (MoCRA 2022) signifies a pivotal shift in the regulatory framework for cosmetic manufacturers, underscoring the critical need for stringent compliance and transparency. New requirements impacting labeling, safety data, adverse event reporting, and Good Manufacturing Practices compel manufacturers to engage proactively with these changes to safeguard consumer safety and trust.
Key insights from MoCRA 2022 reveal updated labeling mandates that necessitate comprehensive ingredient disclosures, enhanced safety data requirements aimed at risk mitigation, and the obligation to report serious adverse events to the FDA. These modifications not only align cosmetic regulations with international safety standards but also mirror the escalating consumer demand for transparency and accountability within the beauty industry. The urgency for compliance is accentuated by the potential penalties for non-adherence, rendering it essential for manufacturers to adopt proactive regulatory strategies.
As the cosmetics industry navigates the implications of MoCRA 2022, it is vital for manufacturers to prioritize compliance and invest in training and education for their teams. By embracing these regulatory changes, companies can fulfill their obligations while positioning themselves as leaders in product safety and consumer trust. The path forward necessitates a commitment to continuous improvement and robust adherence strategies, ensuring that the industry evolves in alignment with consumer expectations and regulatory demands.
Frequently Asked Questions
What is AVS Life Sciences and what services do they provide?
AVS Life Sciences specializes in regulatory compliance solutions, helping manufacturers navigate the complexities of MoCRA 2022. They offer tailored consulting services focused on quality management and regulatory adherence to ensure clients meet updated requirements efficiently.
What are the key changes in labeling requirements under MoCRA 2022?
Key changes include the need for comprehensive ingredient lists, specific usage instructions, necessary warnings, and updated labels reflecting any formulation changes or safety data. By December 29, 2024, labels must also include the contact details of the Responsible Person.
Who is defined as the Responsible Person under MoCRA 2022?
The Responsible Person is defined as the manufacturer, packer, or distributor of a beauty item whose name appears on the product label.
Why is compliance with updated labeling requirements important?
Compliance is crucial to avoid penalties and ensure consumer safety, as well as to build consumer trust and safeguard public health.
What are the enhanced safety data requirements for cosmetic products under MoCRA 2022?
Manufacturers must provide detailed safety information about ingredients, potential risks, and results from safety assessments. This includes implementing robust testing protocols and maintaining thorough documentation.
What are the consequences of non-compliance with safety data requirements?
Non-compliance can lead to serious repercussions, including product recalls and legal consequences, as the FDA has mandatory recall authority over beauty items.
What is the deadline for submitting registration and listing information under MoCRA 2022?
The deadline for submitting registration and listing information is July 1.
What cultural shift is required within organizations due to MoCRA 2022?
Organizations must prioritize safety and transparency in product development, proactively auditing their safety assessment processes and those of their contracted producers and ingredient suppliers to mitigate risks.
