10 Key Benefits of Decentralized Clinical Trials for Compliance Officers

Overview
Decentralized clinical trials (DCTs) present substantial advantages for compliance officers, notably through enhanced patient access, improved data quality, and increased operational efficiency. These benefits are underscored by the way DCTs streamline processes, reduce costs, and uphold regulatory adherence. As a result, DCTs contribute to more successful and inclusive research outcomes. This article details how implementing DCTs not only addresses compliance challenges but also fosters a more effective research environment, ultimately leading to actionable insights for compliance professionals.
Introduction
Decentralized clinical trials are fundamentally reshaping the landscape of medical research, presenting a plethora of advantages that compliance officers must adeptly navigate. By enhancing patient access, improving retention, and enabling real-time data collection, these trials not only streamline processes but also significantly bolster data quality and operational efficiency. However, as the momentum towards decentralized methodologies accelerates, compliance professionals face the pressing question: how can they ensure that regulatory standards are met while fully embracing the innovative potential of these trials? This article delves into ten key benefits that decentralized clinical trials offer to compliance officers, illuminating both the opportunities and challenges that lie ahead.
AVS Life Sciences: Comprehensive Regulatory and Quality Solutions for Decentralized Clinical Trials
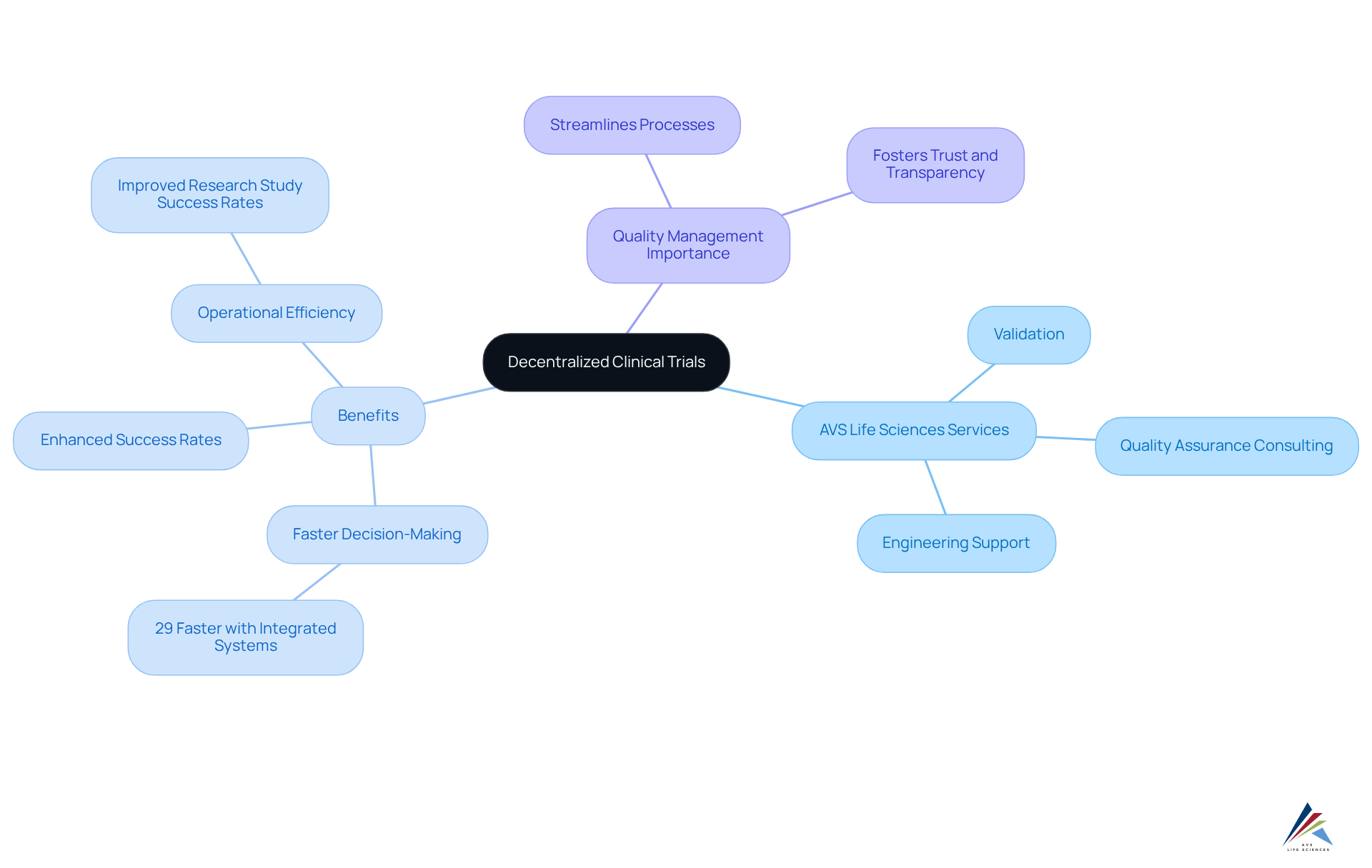
AVS Life Sciences presents a robust framework that highlights the benefits of decentralized clinical trials, ensuring strict compliance with regulatory and quality standards. Their extensive services encompass validation, quality assurance consulting, and engineering support, all of which are critical for maintaining high standards in a decentralized environment.
By harnessing AVS's expertise, regulatory officers can conduct assessments efficiently while ensuring adherence to Good Manufacturing Practices (GMP) and other regulatory requirements. Recent advancements in quality management underscore the importance of integrating comprehensive validation processes, which can significantly boost testing efficiency and compliance outcomes.
Organizations with fully integrated data systems, for instance, experience decision-making that is 29% faster—an essential advantage in the rapidly evolving realm of medical research. Successful case studies involving AVS Life Sciences illustrate how effective quality management strategies contribute to enhanced research study success rates and operational efficiency.
Industry leaders emphasize the pivotal role of quality management in decentralized clinical trials, highlighting the benefits of decentralized clinical trials as it not only streamlines processes but also fosters trust and transparency among stakeholders. By prioritizing quality compliance, AVS Life Sciences solidifies its position as a leader in facilitating decentralized research studies, which underscores the benefits of decentralized clinical trials and ultimately supports the successful delivery of innovative treatments.
Enhanced Patient Access and Engagement in Decentralized Clinical Trials
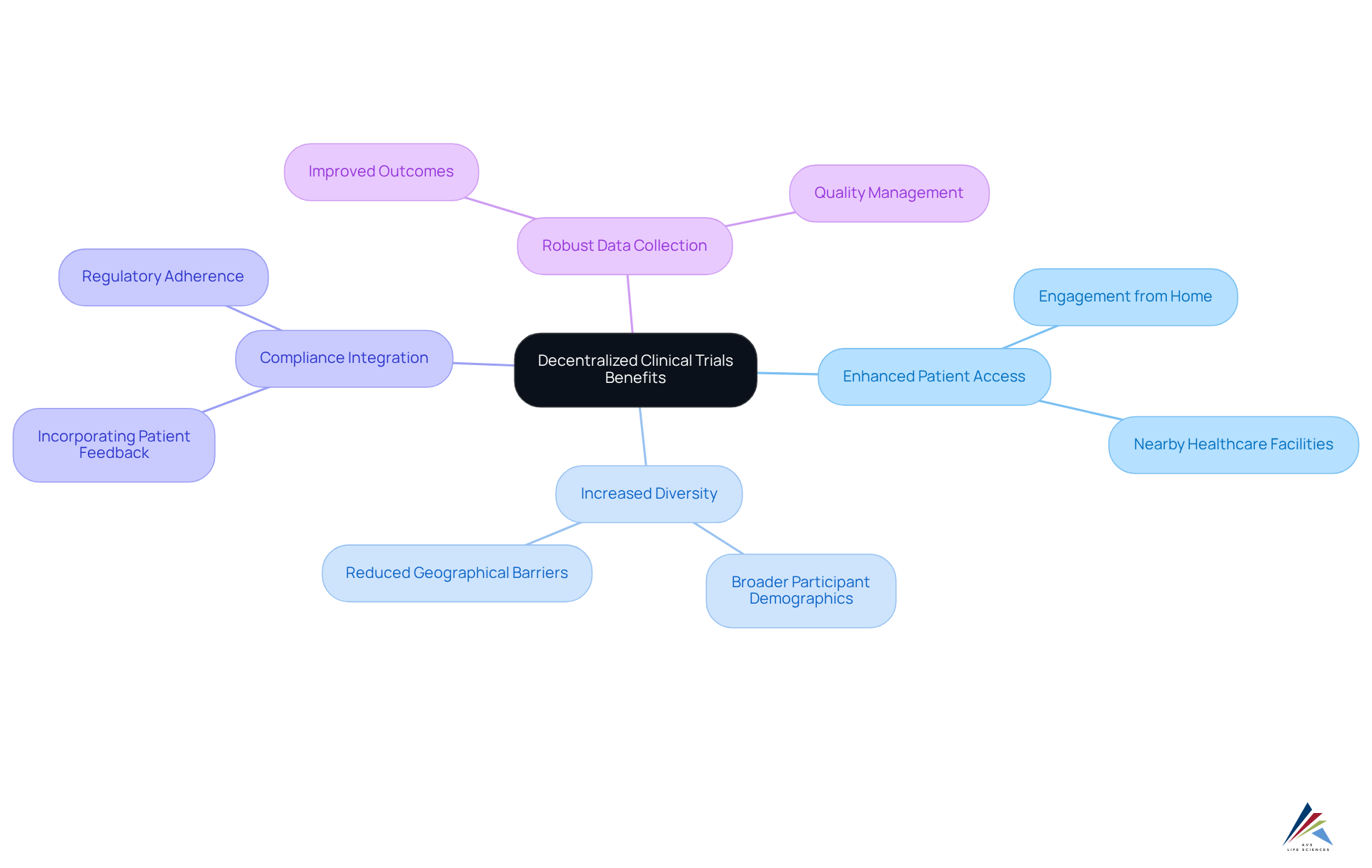
The benefits of decentralized clinical trials significantly enhance patient access, enabling participants to engage from their homes or nearby healthcare facilities. This innovative model effectively diminishes geographical barriers and fosters greater diversity within study populations, showcasing the benefits of decentralized clinical trials.
AVS Life Sciences is resolutely committed to quality management and regulatory adherence. By leveraging this enhanced engagement, compliance officers can seamlessly incorporate patient feedback into the study process. Ultimately, this dedication culminates in more robust data collection and improved outcomes, reinforcing the importance of compliance solutions in clinical research.
Improved Patient Retention in Decentralized Clinical Trials
The benefits of decentralized clinical trials (DCTs) significantly enhance patient retention rates by offering greater flexibility and convenience. By enabling patients to participate without the burden of frequent travel, DCTs address a major obstacle that often disrupts their daily lives. This reduction in travel not only alleviates participant fatigue but also fosters sustained engagement throughout the study, ultimately leading to higher quality data collection.
Statistics reveal that travel-related challenges can result in a 30% decrease in patient retention, underscoring the importance of convenience in study design. Compliance specialists are pivotal in this process, as they can implement strategies that prioritize participant comfort and accessibility. One regulatory specialist noted, 'Reducing travel challenges is crucial; it maintains participants' focus and dedication to the study.'
Furthermore, case studies indicate that studies employing remote monitoring and telehealth options have achieved retention rates exceeding 85%, showcasing the benefits of decentralized clinical trials in maintaining participant engagement. However, it is essential to acknowledge potential challenges, such as the risk of excluding digitally illiterate participants and the necessity for adequate training and support for evaluating DCTs.
By embracing these innovative methods while addressing challenges, regulatory professionals can ensure that studies not only meet legal requirements but also prioritize the needs of participants, resulting in successful outcomes.

Real-Time Data Collection and Analysis in Decentralized Clinical Trials
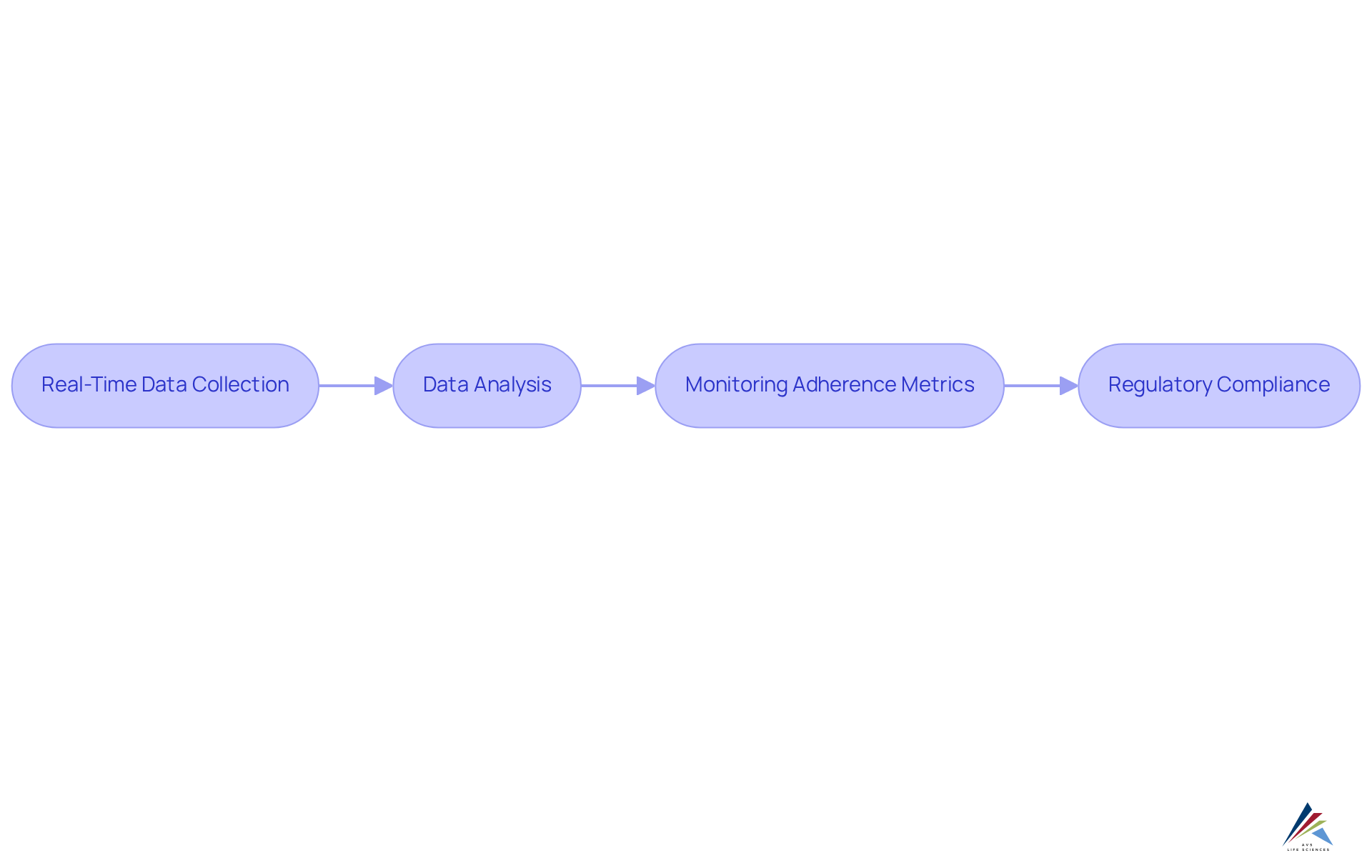
Decentralized clinical studies demonstrate the benefits of decentralized clinical trials by leveraging advanced technologies for real-time data gathering, which facilitates prompt analysis and decision-making. This capability empowers oversight officers to continuously track adherence metrics, ensuring that any deviations from the protocol are swiftly addressed. By adhering to GXP standards and FDA regulations, AVS Life Sciences guarantees that the integration of digital tools not only enhances the overall efficiency of the research process but also upholds stringent quality management practices. This unwavering commitment to comprehensive quality management and regulatory compliance is essential for effective supervision of service providers within the life sciences sector.
Cost-Effectiveness and Operational Efficiency of Decentralized Clinical Trials
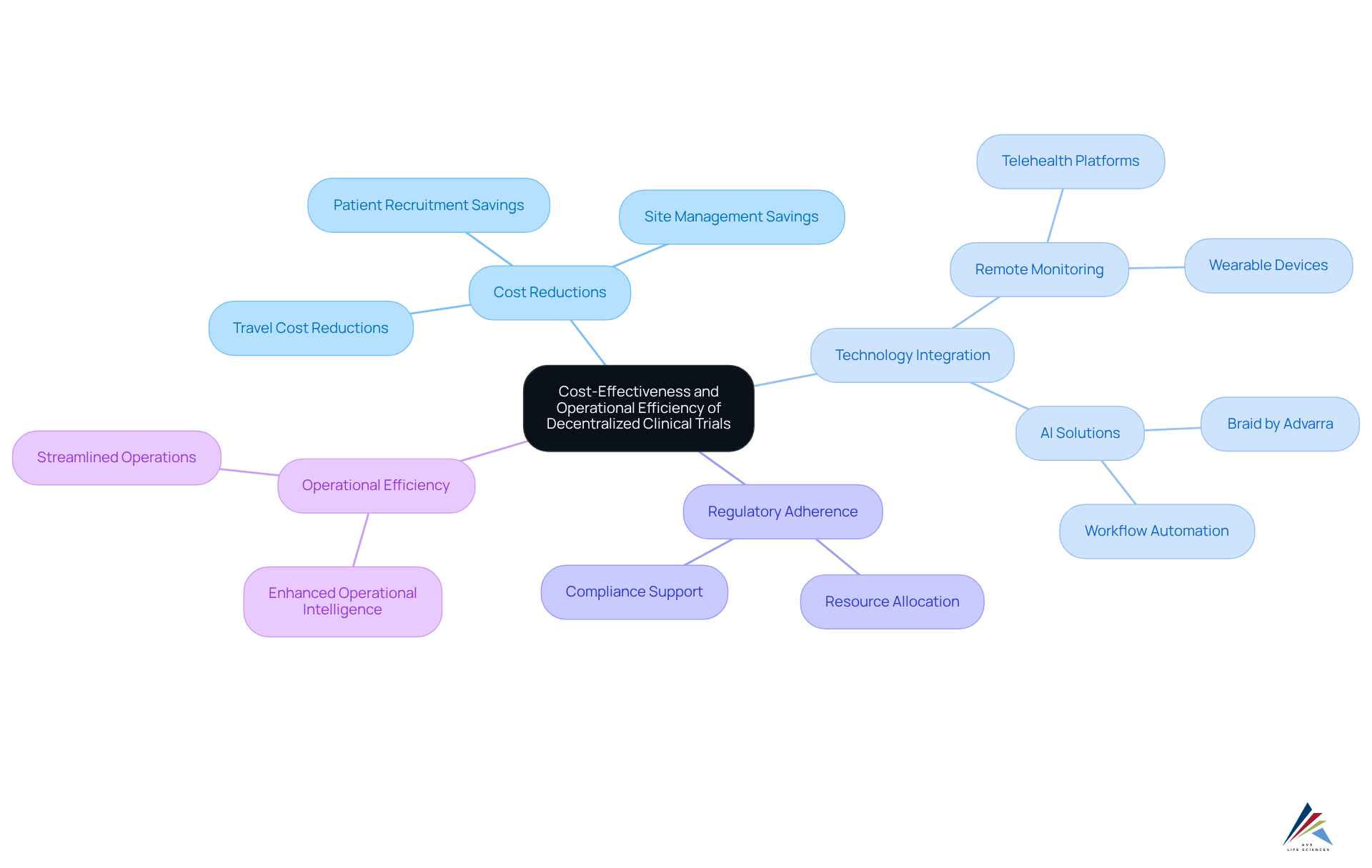
The benefits of decentralized clinical trials include significant reductions in expenses related to patient recruitment, site management, and travel costs. By streamlining operations and leveraging remote monitoring technologies—such as telehealth platforms and wearable devices—organizations can achieve enhanced operational efficiency.
AVS Life Sciences harnesses artificial intelligence to bolster regulatory adherence and quality management within these studies. A prime example is the recent launch of Braid, an AI solution by Advarra, which demonstrates how advanced technology can automate workflows and elevate operational intelligence in research studies.
This innovation not only curtails expenses but also empowers regulatory agents to allocate resources more effectively, ensuring that adherence remains a paramount concern without compromising the study's budget. By integrating AI solutions, AVS Life Sciences supports compliance officers in maintaining rigorous standards of regulatory adherence while refining their research methodologies.
Increased Data Quality and Accuracy in Decentralized Clinical Trials
Decentralized medical studies significantly enhance data quality and precision through standardized digital tools for data gathering. These tools minimize human error, ensuring consistent data capture across all participants.
For instance, the adoption of telemedicine in Phase III studies has surged from 2.7% in 2019 to 5.9% in 2021, reflecting a broader trend towards technology integration in clinical research. A 2021 survey revealed that:
- 94% of research sites had embraced at least one decentralized methodology.
- 88% conducted hybrid trials that blend remote technology with in-person visits.
This transformation not only streamlines data collection but also strengthens the reliability of the information gathered, showcasing the benefits of decentralized clinical trials for compliance officers in supporting regulatory submissions and adhering to industry standards.
The decentralized clinical trial (DCT) market, valued at approximately $6.11 billion in 2020, is projected to reach $16.29 billion by 2027, highlighting the benefits of decentralized clinical trials and the increasing reliance on decentralized strategies in medical research.
Furthermore, around 1,300 drug research studies featuring a virtual or decentralized component were anticipated to commence in 2022, showcasing the benefits of decentralized clinical trials and marking a significant shift towards these innovative methodologies.
The accuracy rates of these digital tools have proven exceptional, further underscoring their role in minimizing discrepancies and enhancing overall data integrity in healthcare studies. Compliance professionals have noted that utilizing these tools is vital for reducing human error in data collection, ensuring that the gathered data meets the highest quality standards.

Faster Recruitment and Study Timelines in Decentralized Clinical Trials
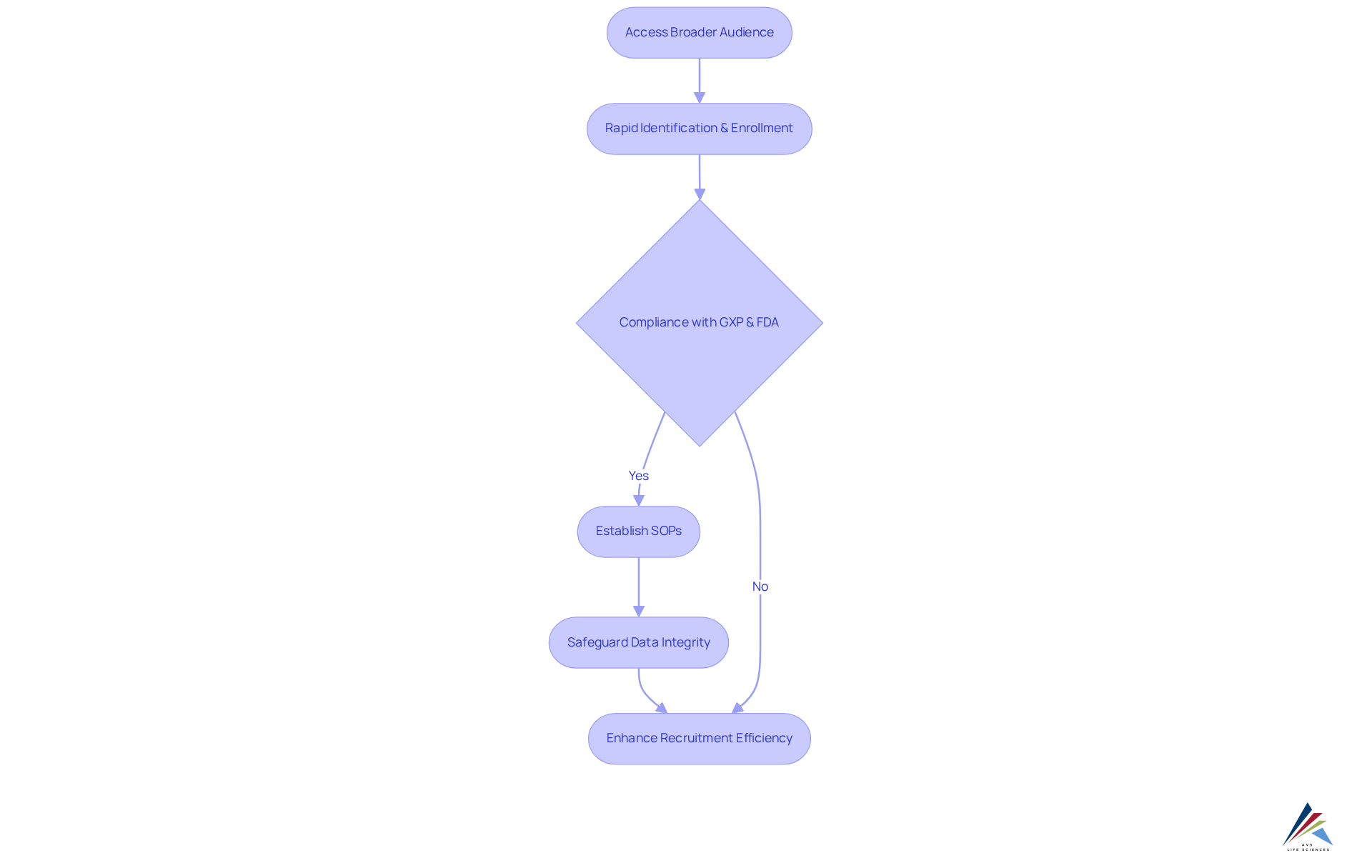
Decentralized medical studies demonstrate the benefits of decentralized clinical trials by enhancing recruitment efficiency through digital platforms that access a broader audience. This innovative approach facilitates the rapid identification and enrollment of eligible participants, showcasing the benefits of decentralized clinical trials in adhering to study timelines.
Compliance officers stand to benefit from these efficiencies, ensuring that evaluations progress as scheduled and remain within regulatory frameworks, particularly by conforming to GXP standards and FDA regulations. By establishing robust Standard Operating Procedures (SOPs) and safeguarding data integrity, compliance measures directly enhance recruitment efficiency, thereby ensuring effective oversight of service providers.
This structured approach not only streamlines processes but also reinforces the critical nature of compliance in the success of decentralized studies.
Adaptability of Decentralized Clinical Trials to External Factors and Disasters
The benefits of decentralized clinical trials provide remarkable flexibility in the face of external challenges, such as natural disasters and public health crises. By facilitating remote participation, these studies ensure continuity without significant interruptions. This adaptability is vital for compliance officers, who can utilize it to ensure that the benefits of decentralized clinical trials are achieved while examinations remain compliant and operational even in adverse conditions.
For instance, during the COVID-19 pandemic, numerous studies successfully transitioned to remote formats, allowing them to continue despite widespread disruptions. This transition not only preserved the integrity of the studies but also enhanced participant engagement by reducing geographical barriers.
Compliance professionals recognize that such flexibility is essential for effectively managing assessments during emergencies, as it allows for rapid adjustments to protocols while maintaining adherence to regulatory standards.
AVS Life Sciences provides comprehensive quality management and regulatory compliance solutions that support compliance officers in navigating the complexities of decentralized studies. The ability to swiftly adapt to external influences underscores the benefits of decentralized clinical trials in the context of today’s dynamic research landscape.

Ethical Considerations and Compliance in Decentralized Clinical Trials
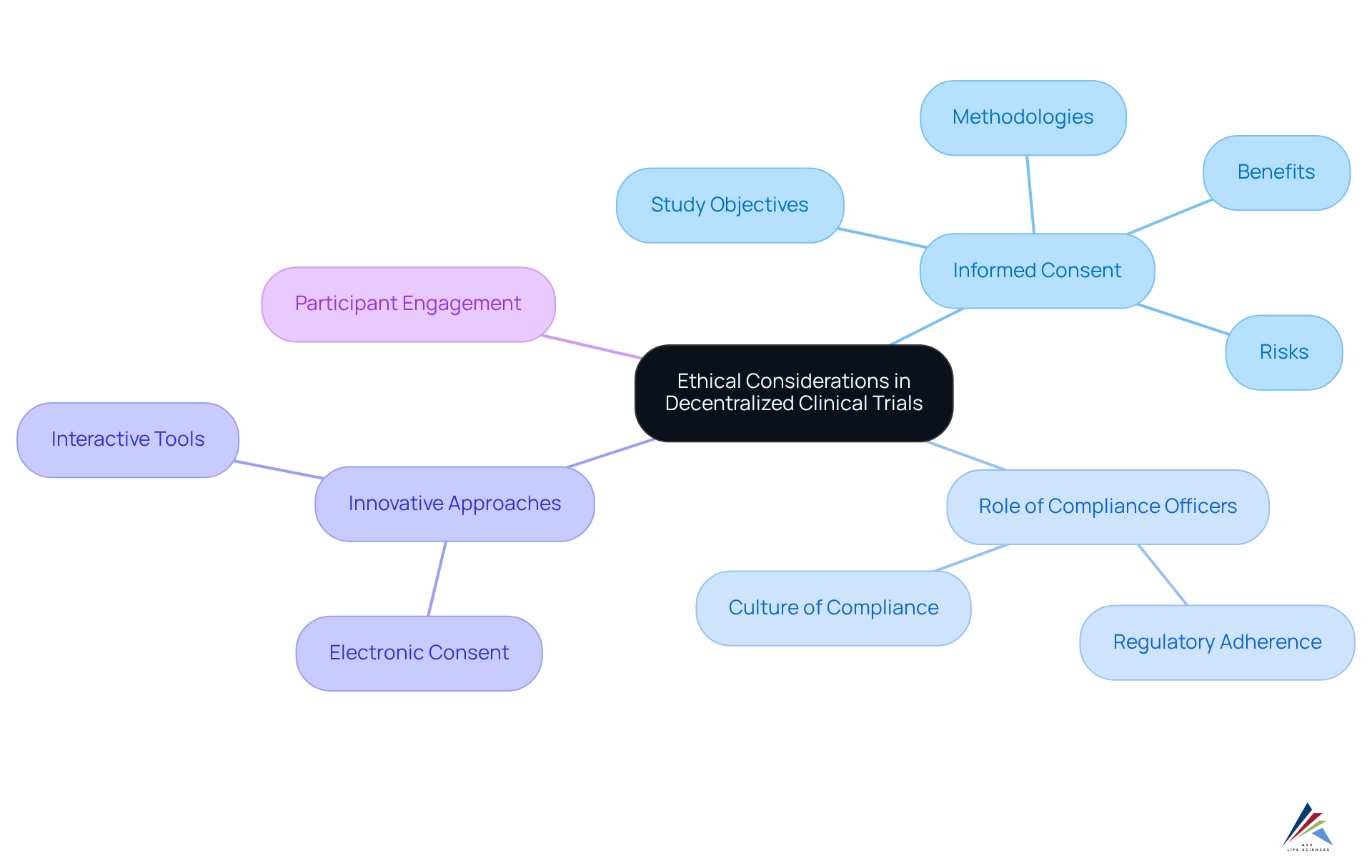
Decentralized clinical studies (DCTs) operate within a framework of stringent ethical standards that safeguard participant rights and ensure informed consent. Compliance officers play a critical role in this environment, charged with the responsibility of overseeing adherence to regulatory mandates and ethical benchmarks. Their involvement is essential in cultivating a culture of ethical compliance, which not only enhances trust but also promotes transparency throughout the study process.
Informed consent in DCTs requires that participants receive thorough information regarding the study's objectives, methodologies, risks, and potential benefits, empowering them to make informed decisions. This aspect is particularly crucial in remote studies, where participants may engage from the comfort of their homes through telemedicine and mobile health applications.
Recent dialogues among regulatory officers underscore the urgent need for innovative approaches to informed consent, highlighting the importance of accessibility and active participant engagement. For example, the adoption of electronic consent (e-consent) platforms can simplify the process, rendering it more user-friendly while upholding the integrity of the information shared.
Effective informed consent practices in remote studies often involve interactive tools that facilitate real-time dialogue between participants and research staff, ensuring clarity and understanding. Such methodologies not only adhere to ethical standards but also empower participants, reinforcing their autonomy and active involvement in the research process.
By prioritizing informed consent and ethical standards, organizations can adeptly navigate the complexities inherent in decentralized studies, highlighting the benefits of decentralized clinical trials while steadfastly protecting the rights and well-being of participants.
Transformative Impact of Decentralized Clinical Trials on Future Research
Decentralized clinical studies (DCTs) are set to revolutionize the future of clinical research by demonstrating the benefits of decentralized clinical trials, enhancing inclusivity and operational efficiency. As these models gain traction, compliance officers must adapt their strategies to effectively navigate the evolving regulatory landscape. The transition to decentralized studies not only streamlines processes but also fosters innovation within the research environment.
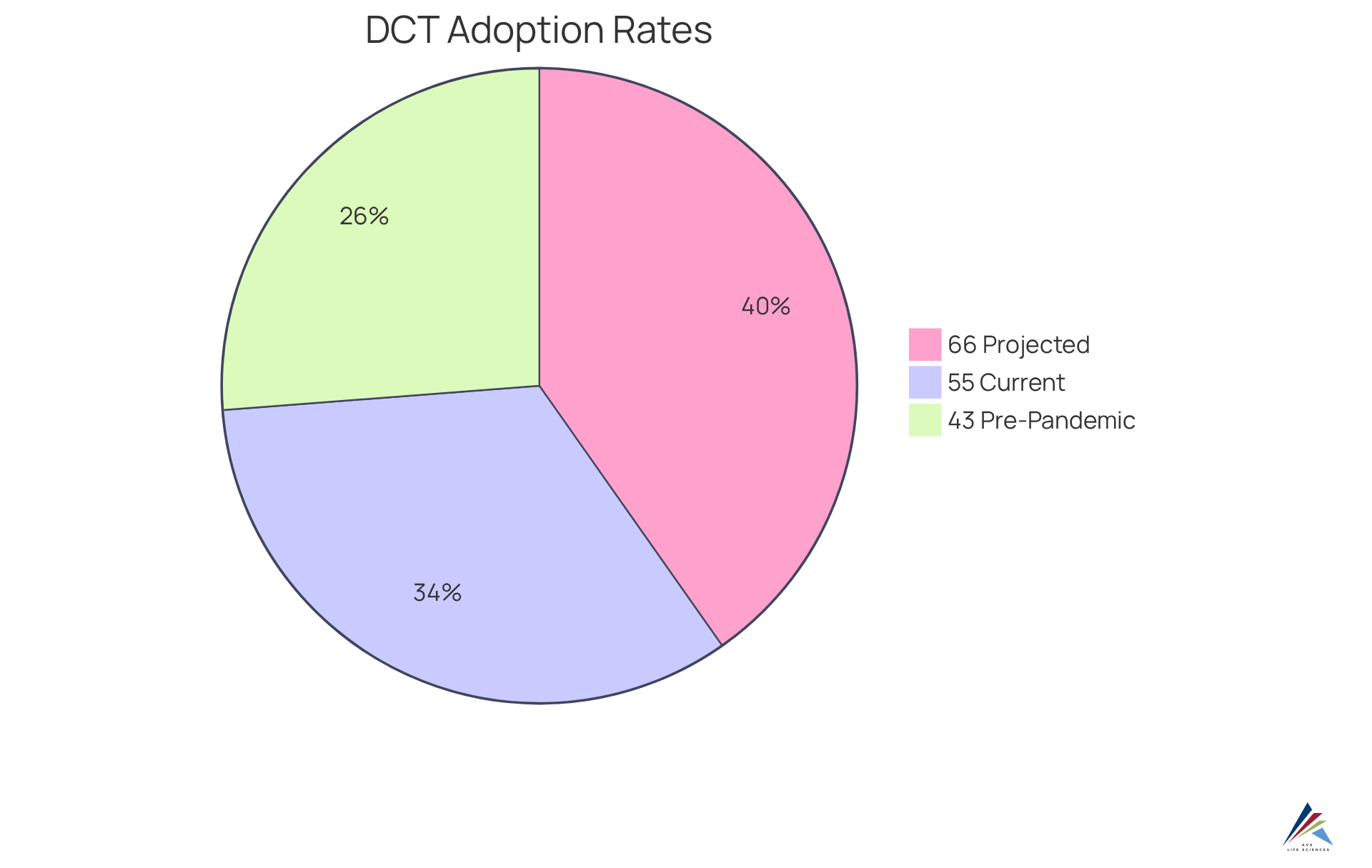
Notably, the average number of studies utilizing decentralized technologies has escalated from 43% pre-pandemic to 55% currently, with projections suggesting an increase to 66% within the next five years. This trend underscores the growing recognition of DCTs as a vital component of research portfolios, with 100% of biopharmaceuticals and contract research organizations (CROs) anticipating their significance by the end of 2020.
Furthermore, the benefits of decentralized clinical trials include an expected reduction in study costs by 10-25%, primarily through fewer locations, lower investigator fees, and decreased patient travel expenses, making them an attractive option for organizations aiming to optimize resources while ensuring compliance.
Industry leaders assert that the benefits of decentralized clinical trials show they are not merely a passing trend but a critical evolution for enhancing patient engagement and inclusivity. As the landscape continues to transform, the capacity to adapt and leverage these innovative approaches will be essential for compliance officers dedicated to ensuring regulatory adherence and advancing research excellence.
Conclusion
Decentralized clinical trials (DCTs) signify a transformative shift in the clinical research landscape, presenting numerous advantages that enhance patient engagement, streamline processes, and ensure adherence to regulatory standards. By adopting this innovative approach, compliance officers can markedly elevate the efficiency and effectiveness of clinical studies, ultimately resulting in improved patient outcomes and expedited delivery of new treatments.
This article has highlighted the key benefits of decentralized trials, such as:
- Enhanced patient access
- Improved retention rates
- Real-time data collection
- Cost-effectiveness
- Adaptability to external challenges
Each of these elements contributes to a more robust and inclusive research environment, where compliance professionals are essential in navigating the complexities of regulatory adherence and ethical considerations.
As the reliance on decentralized clinical trials continues to expand, it is imperative for organizations to adjust their strategies to fully leverage these methodologies. By prioritizing quality management and regulatory compliance, stakeholders can ensure the successful implementation of DCTs, paving the way for a future where clinical research is more efficient, accessible, and aligned with the diverse needs of patient populations. Embracing these changes not only enhances research outcomes but also reinforces the commitment to ethical standards and participant welfare, marking a significant advancement in the field of clinical research.
Frequently Asked Questions
What are the main benefits of decentralized clinical trials (DCTs) according to AVS Life Sciences?
Decentralized clinical trials enhance patient access by allowing participants to engage from their homes or nearby healthcare facilities, diminish geographical barriers, foster greater diversity within study populations, and improve patient retention rates by offering flexibility and convenience.
How does AVS Life Sciences ensure compliance in decentralized clinical trials?
AVS Life Sciences ensures compliance by providing a robust framework that includes validation, quality assurance consulting, and engineering support, which are critical for maintaining high standards and adherence to Good Manufacturing Practices (GMP) and other regulatory requirements.
What impact do integrated data systems have on decision-making in clinical research?
Organizations with fully integrated data systems experience decision-making that is 29% faster, which is a significant advantage in the rapidly evolving realm of medical research.
How do decentralized clinical trials improve patient retention?
Decentralized clinical trials improve patient retention by reducing travel burdens, which can lead to a 30% decrease in retention rates. By enabling patients to participate without frequent travel, DCTs maintain participant engagement and lead to higher quality data collection.
What role do compliance specialists play in decentralized clinical trials?
Compliance specialists implement strategies that prioritize participant comfort and accessibility, ensuring that studies meet legal requirements while addressing the needs of participants to achieve successful outcomes.
What challenges might arise with decentralized clinical trials?
Potential challenges include the risk of excluding digitally illiterate participants and the necessity for adequate training and support for evaluating decentralized clinical trials.
What are the outcomes of studies employing remote monitoring and telehealth options in decentralized clinical trials?
Studies that employ remote monitoring and telehealth options have achieved retention rates exceeding 85%, demonstrating the effectiveness of decentralized clinical trials in maintaining participant engagement.
