10 Essential Steps in the Computer System Validation Lifecycle

Overview
The primary focus of this article is to delineate the essential steps involved in the computer system validation (CSV) lifecycle, a crucial process that ensures compliance and operational integrity within regulated environments. It meticulously details critical phases such as:
- Planning
- Requirements definition
- Risk assessment
- Documentation standards
Each phase is interconnected, underscoring the necessity of adhering to regulatory guidelines, which ultimately enhances product quality and safeguards patient safety.
Introduction
The intricate landscape of the pharmaceutical and biotechnology industries necessitates rigorous adherence to regulatory standards, particularly regarding computer system validation (CSV). This essential process not only guarantees that systems function as intended but also protects product quality and patient safety amidst the growing scrutiny and complexity in biopharmaceuticals.
As organizations confront the challenge of navigating the CSV lifecycle, it is imperative to explore the essential steps that enhance compliance and operational integrity.
What are the key phases and best practices that can elevate validation from a mere requirement to a strategic advantage?
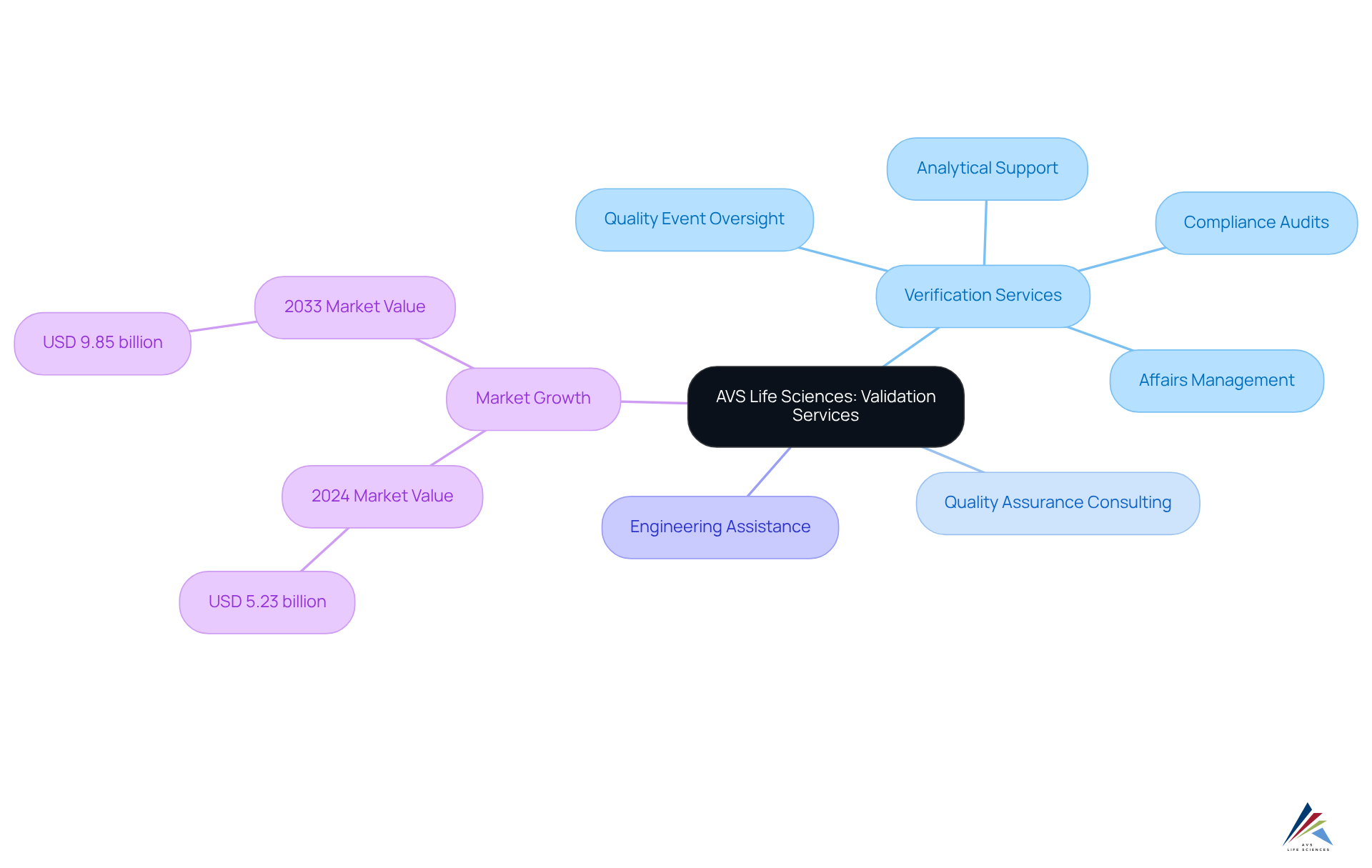
AVS Life Sciences: Comprehensive Validation Services for Lifecycle Management
AVS Life Sciences offers a comprehensive suite of verification services tailored for the pharmaceutical and biotechnology sectors. These services encompass verification and commissioning, quality assurance consulting, and engineering assistance, all meticulously designed to ensure that clients adhere to stringent legal standards throughout the product lifecycle. This includes essential services such as analytical support, affairs management, quality event oversight, and , which are vital for maintaining quality and compliance during the drug development process.
The significance of these services is highlighted by the projected growth of the Global Pharmaceutical Validation Services Market, expected to rise from USD 5.23 billion in 2024 to USD 9.85 billion by 2033, representing a compound annual growth rate (CAGR) of 7.3% from 2026 to 2033. This expansion is driven by increasing regulatory scrutiny and the growing complexity of biopharmaceuticals, underscoring the need for specialized verification expertise.
AVS Life Sciences distinguishes itself as a trusted partner, leveraging innovation and a client-centric approach to navigate the complex validation processes. Their unwavering commitment to quality and compliance not only enhances operational efficiency but also equips clients for success in a competitive landscape. Engaging with AVS Life Sciences means embracing a future where compliance is not just a requirement but a pathway to excellence.
Define Computer System Validation: Key Concepts and Importance
The computer system validation lifecycle is a structured and recorded procedure that ensures a computing setup reliably meets its intended functions. This process is crucial for upholding adherence to standards, particularly FDA 21 CFR Part 11, which oversees electronic records and signatures. By ensuring data integrity, CSV plays a pivotal role in mitigating risks associated with software failures, thereby protecting product quality and patient safety.
The verification process in the computer system validation lifecycle includes:
- Thorough testing
- Detailed documentation
- Incorporating compliance with Good Practice (GXP) standards
- Creation of Standard Operating Procedures (SOPs)
These steps ensure that systems function as intended—a necessity in the highly regulated life sciences sector.
As the market for CSV is projected to grow significantly, with an expected value of USD 14.02 billion by 2037, the importance of adhering to these standards cannot be overstated. Real-world examples demonstrate that organizations adopting strong CSV practices not only boost adherence to FDA guidelines but also enhance operational efficiency and decrease the chances of expensive penalties.
To effectively navigate the complexities of the computer system validation lifecycle, organizations should prioritize incorporating into their verification processes, ensuring a thorough approach to quality management and regulatory adherence.

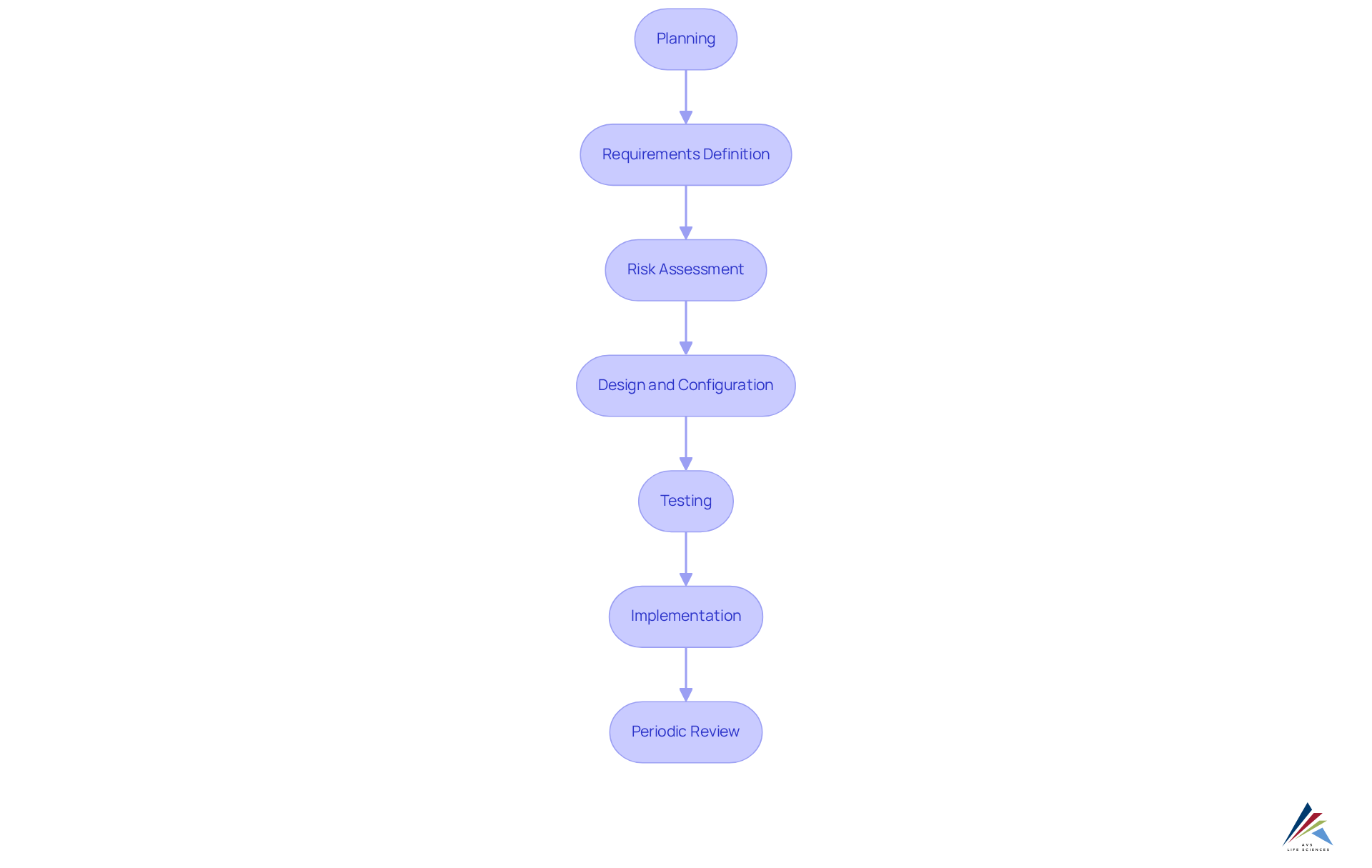
Outline the Phases of the Computer System Validation Lifecycle
The computer system validation lifecycle encompasses several critical phases that ensure compliance and operational integrity in pharmaceutical environments. These phases include:
- Planning: Creating a master plan for verification that outlines the scope, objectives, responsibilities, and resources, ensuring a comprehensive approach to assessment. Engaging with vendors early in the process can provide valuable insights into their validation support.
- Requirements Definition: Identifying user needs and functional specifications (URS and FRS), which are essential for aligning the setup with stakeholder expectations.
- Risk Assessment: Evaluating potential risks linked to the framework, focusing on their impact on product quality, data integrity, and patient safety. This phase should prioritize based on identified risks.
- Design and Configuration: Developing the framework according to the established specifications, ensuring that all components meet regulatory standards.
- Testing: Conducting installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) to validate that the system operates as intended under real-world conditions. This multi-step process is essential, as it permits quality checks after each verification step, ensuring adherence to FDA regulations and GXP standards. The FDA defines software validation as "Confirmation, by inspection and the presentation of tangible evidence, that the software specifications comply with the purposes and planned uses of the user and that the specific requirements implemented through software can consistently meet their obligations."
- Implementation: Launching the validated setup into production, with suitable procedures and training established to guarantee adherence.
- Periodic Review: Regularly evaluating the framework to uphold continuous compliance and performance, which is vital for adjusting to changing regulatory requirements. Additionally, establishing a comprehensive audit trail is critical for monitoring data integrity throughout the lifecycle.
Each phase of the computer system validation lifecycle is interconnected, making careful documentation and adherence to best practices essential to ensure the validated state of the framework throughout its lifecycle. Frequent revisions to training resources are essential to conform with changing regulations and best practices, and a retirement strategy for the framework should be created to guarantee proper decommissioning at the conclusion of its useful life.
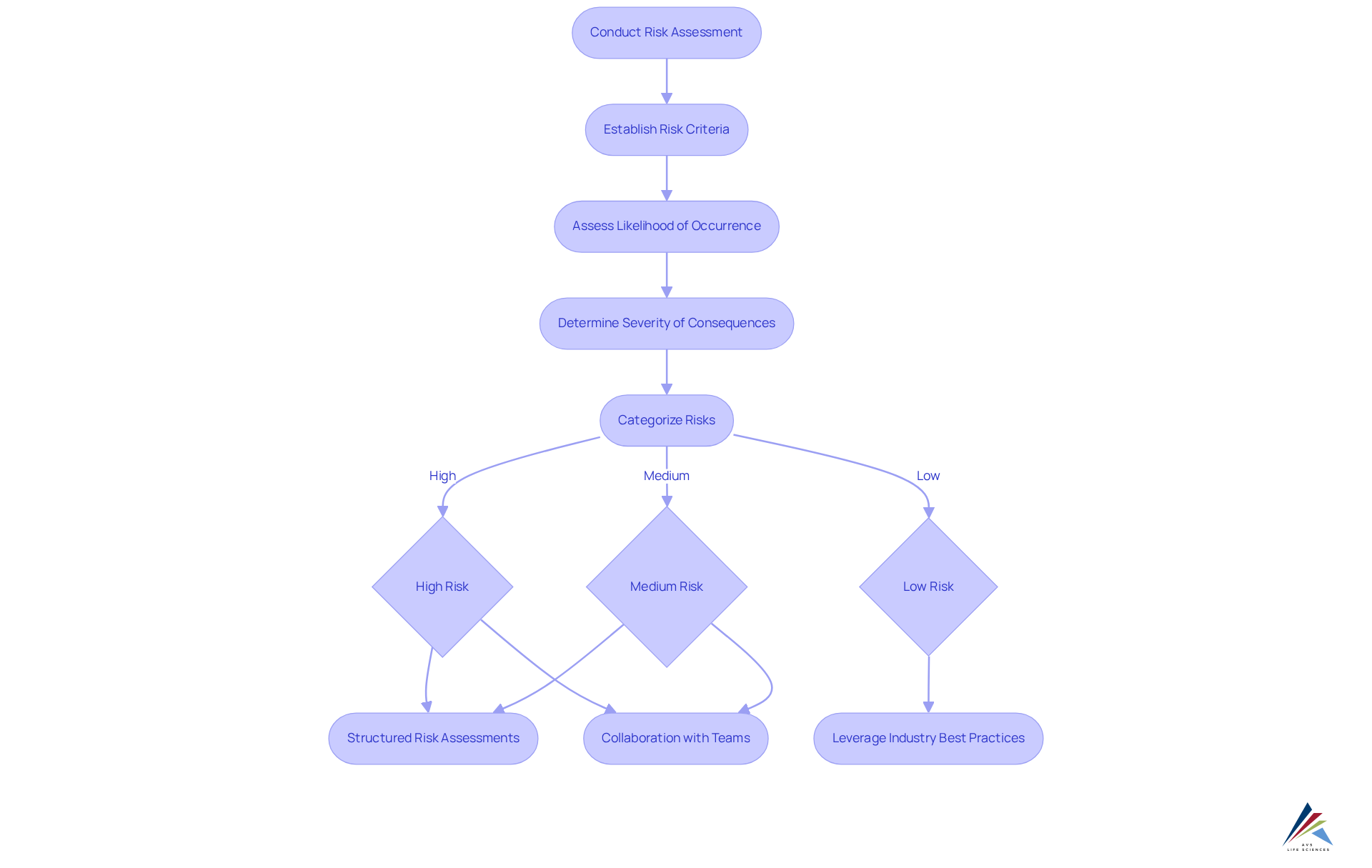
Conduct Risk Assessment: Identifying and Mitigating Potential Issues
Conducting a comprehensive risk assessment is crucial for identifying potential hazards associated with the computer system validation lifecycle and evaluating their operational impact. This process involves establishing clear risk criteria, assessing the likelihood of occurrence, and determining the severity of potential consequences. Risks should be categorized into high, medium, and low based on their impact on data integrity, patient safety, or product quality. By prioritizing risks, organizations can effectively allocate resources to address issues proactively, thereby improving adherence and simplifying the assessment process. This guarantees that vital areas receive concentrated attention, ultimately enhancing success rates in assessment.
Effective methods for identifying risks include:
- Structured risk assessments
- Collaboration with cross-functional teams
- Leveraging industry best practices
Involving all relevant departments from the earliest stages of a project can prevent last-minute changes. Furthermore, employing strong risk management strategies, such as regular audits and ongoing monitoring, can greatly diminish the chances of regulatory failures and improve overall system integrity. The FDA's guidance for Process Validation emphasizes the importance of the computer system validation lifecycle, recommending the use of sound statistical methods for continuous process verification and further supporting the need for a structured risk assessment approach.
For organizations looking for expert assistance in this field, AVS Life Sciences provides extensive support and resources to ensure successful Computer System Confirmation, including a detailed checklist to direct your confirmation projects.
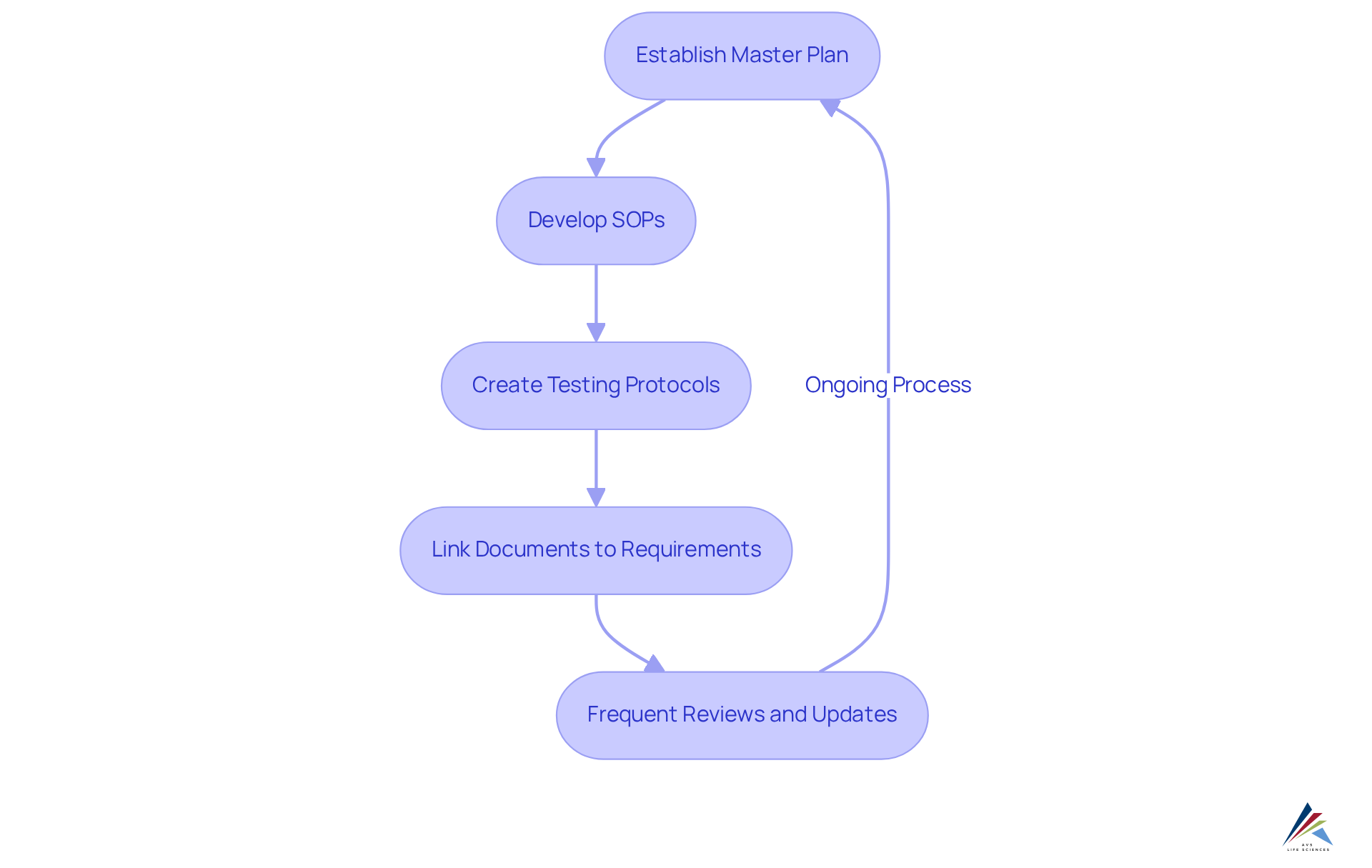
Establish Documentation Standards: Ensuring Compliance and Traceability
Establishing robust documentation standards is vital for ensuring compliance with regulatory requirements in the life sciences sector. This necessity entails the development and upkeep of a master plan for verification, standard operating procedures (SOPs), and detailed testing protocols. Each document must clearly outline the validation process, including objectives, methodologies, and results.
Traceability is paramount; all documentation should link back to , demonstrating that the system meets its intended use. In fact, 83% of risk and regulatory professionals view upholding adherence to laws, policies, and regulations as crucial in their decision-making processes. Proper documentation not only facilitates audits but also supports continuous improvement efforts.
Organizations with effective documentation practices can mitigate risks and reduce the likelihood of audit findings. Optimal methods in compliance documentation involve frequent reviews and updates to guarantee alignment with changing standards. Successful execution of SOPs in the computer system validation lifecycle can significantly enhance traceability, ensuring that all verification activities are documented and easily retrievable, thereby reinforcing the integrity of the verification process.
Implement Training Programs: Ensuring Competency in Validation Practices
Establishing thorough training initiatives is crucial for ensuring that staff engaged in the assessment process possess the necessary skills and understanding of compliance standards and optimal methods. Key topics for training should encompass:
- Risk assessment
- Documentation standards
- Change control procedures
Regular refresher courses and hands-on workshops are essential for reinforcing skills and keeping staff informed about the latest industry developments. Organizations that foster a culture of not only enhance their verification efforts but also significantly improve adherence to evolving regulations. In fact, companies with robust training programs report a 17% increase in productivity and a 218% higher income per employee compared to those lacking formalized training. By prioritizing employee development, organizations can adeptly navigate the complexities of regulatory adherence, ensuring that their validation practices meet the highest standards.

Manage Changes: Implementing Effective Change Control Procedures
Implementing robust change control procedures is essential for preserving the integrity of validated frameworks within the computer system validation lifecycle in the life sciences. Organizations must establish a formal that includes a comprehensive impact assessment of proposed modifications within the computer system validation lifecycle. This involves documenting expected outcomes and conducting rigorous testing prior to implementation. Effective communication with stakeholders is crucial, ensuring that all relevant parties are informed and engaged throughout the process. By adhering to a strict change control framework, organizations can protect the validated condition of their processes within the computer system validation lifecycle, thus ensuring continuous adherence and operational excellence.
The stages of the computer system validation lifecycle—including Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ)—emphasize the necessity of thorough testing and documentation in preserving integrity. As noted by industry leaders, the cost of stagnation often outweighs the cost of change, making it imperative for organizations to embrace structured change management practices to thrive in a competitive landscape.
AVS Life Sciences illustrates this dedication through successful initiatives, such as the enhancement of a biotechnology GMP facility, where effective change control and quality assurance were crucial in attaining adherence and operational success. By adopting these practices, organizations not only comply with regulatory standards but also position themselves for sustained growth and excellence in the life sciences sector.
Conduct Periodic Reviews: Maintaining Compliance Over Time
Frequent evaluations of computer networks are indispensable for maintaining their verified condition and ensuring compliance with legal requirements. Periodic reviews empower organizations to assess system performance, confirm adherence to regulatory standards, and identify any modifications since the last evaluation. Establishing a well-organized timetable for these assessments is crucial, with documentation serving as a fundamental component of compliance. Statistics indicate that numerous regulatory issues surface during these reviews, underscoring the and corrective actions to mitigate risks. By implementing effective strategies for sustaining compliance over time, organizations can protect product quality and patient safety, thereby reinforcing their commitment to industry standards.

Leverage Technology: Enhancing Validation Through Automation
Leveraging technology, particularly automation tools, significantly enhances the efficiency of the validation process. AVS Life Sciences exemplifies this by incorporating automated batch reporting solutions that consolidate key process parameters. This innovation enables operators to focus on vaccine production rather than . Automated systems streamline documentation, testing, and reporting, effectively reducing the time and effort required for manual tasks.
For instance, organizations employing automation have noted up to a 50% reduction in testing time alongside a substantial decline in errors—an essential factor for maintaining regulatory compliance. Furthermore, automation enhances data accuracy and consistency, thereby minimizing the risk of human error.
By integrating advanced technologies into the verification lifecycle, such as transitioning from a Biosafety Level 1 to a Level 2 GMP facility, organizations can refine their processes, ensure adherence to regulations, and concentrate on strategic initiatives that foster innovation.

Understand Regulatory Requirements: Navigating Compliance Standards
Grasping compliance obligations is essential for effective computer system verification. Organizations must stay informed about relevant regulations, including FDA guidelines, ISO standards, and GxP requirements. Consistent training and updates on rule changes are vital for guaranteeing adherence; firms that implement a strong computer system validation lifecycle have observed a remarkable 35% enhancement in adherence rates. By proactively addressing compliance expectations, organizations can mitigate risks associated with non-conformity and enhance their overall efforts within the computer system validation lifecycle.
As the Computer System Validation market is projected to reach $9,457.96 million by 2032, the growing significance of adherence in the industry cannot be overstated. Engaging with oversight entities and industry associations offers valuable insights into best practices and emerging trends, assisting organizations in navigating the complexities of diverse regulations. To further strengthen their compliance efforts, organizations should consider establishing and participating in industry forums to share knowledge and strategies.
Partnering with AVS Life Sciences can provide expert guidance and comprehensive consulting services, ensuring that organizations are well-equipped to meet regulatory demands and achieve successful validation outcomes.
Conclusion
The computer system validation lifecycle represents a crucial framework that guarantees the reliability and integrity of computing systems within the highly regulated pharmaceutical and biotechnology sectors. By adhering to structured processes and stringent standards, organizations can effectively mitigate risks, enhance compliance, and ultimately safeguard product quality and patient safety.
Key steps in the validation lifecycle have been outlined, including:
- Planning
- Risk assessment
- Documentation
- The critical importance of implementing robust training and change control procedures
Each phase plays a vital role in maintaining a validated state, ensuring that systems not only meet regulatory requirements but also operate efficiently in real-world conditions. The emphasis on leveraging technology and automation further underscores the potential for increased accuracy and reduced operational burdens.
In a landscape where regulatory scrutiny is intensifying and the stakes are high, the significance of a comprehensive approach to computer system validation cannot be overstated. Organizations are strongly encouraged to prioritize ongoing education, engage with industry best practices, and consider partnering with experts like AVS Life Sciences to navigate the complexities of compliance. By doing so, they can not only meet current standards but also position themselves for future success in a rapidly evolving industry.
Frequently Asked Questions
What services does AVS Life Sciences offer?
AVS Life Sciences offers a comprehensive suite of verification services tailored for the pharmaceutical and biotechnology sectors, including verification and commissioning, quality assurance consulting, and engineering assistance.
Why are the services provided by AVS Life Sciences important?
These services are crucial for ensuring adherence to stringent legal standards throughout the product lifecycle, maintaining quality and compliance during the drug development process, and addressing increasing regulatory scrutiny in the industry.
What is the projected growth of the Global Pharmaceutical Validation Services Market?
The market is expected to grow from USD 5.23 billion in 2024 to USD 9.85 billion by 2033, with a compound annual growth rate (CAGR) of 7.3% from 2026 to 2033.
What is computer system validation (CSV)?
Computer system validation is a structured procedure that ensures a computing setup reliably meets its intended functions, crucial for adhering to standards such as FDA 21 CFR Part 11, which oversees electronic records and signatures.
What are the key components of the computer system validation lifecycle?
The key components include thorough testing, detailed documentation, compliance with Good Practice (GXP) standards, and the creation of Standard Operating Procedures (SOPs).
What are the phases of the computer system validation lifecycle?
The phases include: - Planning - Requirements Definition - Risk Assessment - Design and Configuration - Testing - Implementation - Periodic Review
What is the significance of the testing phase in the computer system validation lifecycle?
The testing phase includes installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ), ensuring that the system operates as intended and adheres to FDA regulations and GXP standards.
How does the computer system validation lifecycle contribute to product quality and patient safety?
By ensuring data integrity and compliance with regulatory standards, the CSV lifecycle mitigates risks associated with software failures, thereby protecting product quality and patient safety.
What should organizations prioritize to effectively navigate the complexities of the computer system validation lifecycle?
Organizations should prioritize incorporating GXP standards into their verification processes to ensure a thorough approach to quality management and regulatory adherence.
Why is documentation important in the computer system validation lifecycle?
Careful documentation is essential to ensure the validated state of the framework throughout its lifecycle and to maintain compliance with changing regulations and best practices.
