10 Essential Steps for GDUFA Self Identification Compliance
Overview
The primary objective of this article is to delineate the crucial steps necessary for compliance with GDUFA self-identification. It underscores the imperative for organizations engaged in the production of generic drugs to adhere to specific regulatory requirements. Failure to do so may result in significant penalties and jeopardize market access. The article provides comprehensive guidance on the submission process, effective documentation management, and the repercussions of non-compliance, ensuring that stakeholders are well-informed and prepared to navigate the complexities of regulatory adherence.
Introduction
Navigating the intricate landscape of regulatory compliance presents a formidable challenge for organizations engaged in the production of generic medications. The Generic Drug User Fee Amendments (GDUFA) impose stringent self-identification requirements that are critical for maintaining market access and avoiding severe penalties. This article delineates ten essential steps for achieving GDUFA self-identification compliance, equipping readers with the knowledge necessary to successfully maneuver through the complexities of regulatory obligations.
What are the implications if an organization fails to comply, and how can they ensure adherence to these vital standards?
AVS Life Sciences: Comprehensive Regulatory Solutions for GDUFA Compliance
AVS Life Sciences presents a comprehensive suite of services tailored to help organizations navigate the complexities of the Generic Drug User Fee Amendments, focusing on gdufa self identification. Their multifaceted approach encompasses:
- Validation
- Quality assurance consulting
- Engineering assistance
This enables clients to adeptly traverse the intricate regulatory landscape. With a steadfast commitment to innovation and expertise, AVS Life Sciences empowers clients to maintain rigorous throughout the product lifecycle. This dedication has established AVS as a trusted partner in the pharmaceutical sector, particularly as regulatory compliance becomes increasingly vital for maintaining a competitive advantage and securing market access.
The impact of legislation on adherence strategies is profound, necessitating user fees and emphasizing the importance of timely inspections and data transparency. AVS effectively addresses these challenges through its tailored solutions. For instance, successful case studies from life sciences consulting firms underscore the critical role of proactive compliance measures, further solidifying AVS's status as an industry leader in compliance strategies. By engaging with AVS Life Sciences, organizations can not only meet regulatory demands but also enhance their operational efficiency and achieve gdufa self identification in the market.
Self-Identification Requirements: Who Must Comply with GDUFA?
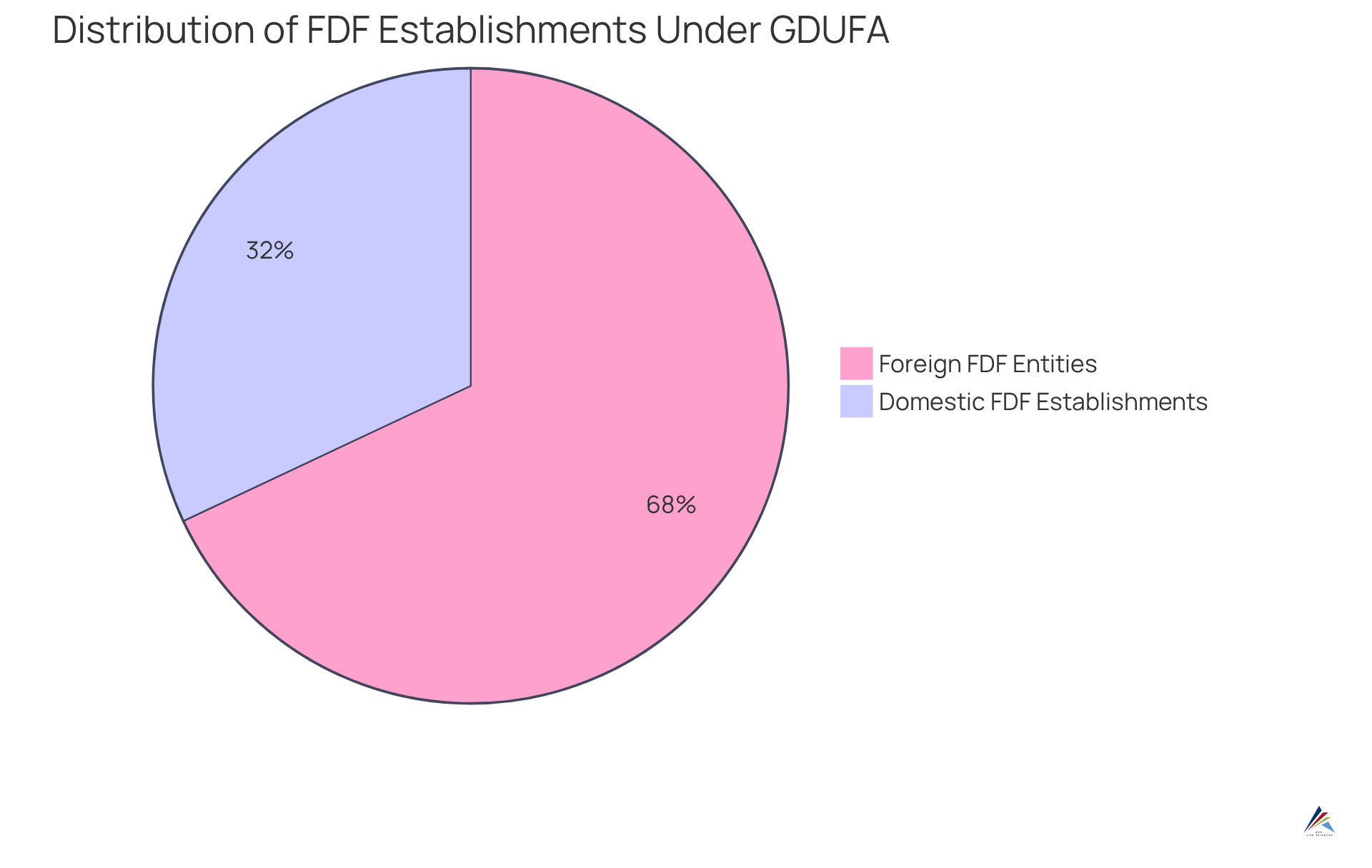
Under GDUFA, all establishments involved in the production of human generic medications, including Active Pharmaceutical Ingredients (APIs) and Finished Dosage Forms (FDFs), must complete with the FDA. This requirement encompasses both domestic and international locations, ensuring the FDA maintains an accurate inventory of entities engaged in generic drug production. Compliance is crucial—not only for maintaining eligibility for user fees but also for avoiding regulatory complications that could arise from non-compliance.
Statistics indicate that there are:
- 153 domestic FDF establishments
- 325 foreign FDF entities
that must adhere to these requirements. Furthermore, sites performing testing of attributes or characteristics of FDFs or APIs to satisfy [Current Good Manufacturing Practices](https://avslifesciences.com/industry-news/cders-quality-management-maturity-program) (cGMP) must also conduct GDUFA self identification. Obstacles in adherence frequently stem from the intricacies of precisely documenting operations and ensuring that all pertinent activities are included in the GDUFA self identification process.
As highlighted by FDA representatives, failure to self-identify can result in serious repercussions, including the possible misbranding of all products produced at the site. This underscores the critical nature of compliance for all stakeholders in the generic drug manufacturing landscape. To navigate these complexities effectively, it is essential for establishments to engage with compliance solutions that facilitate accurate self-identification and adherence to regulatory standards.
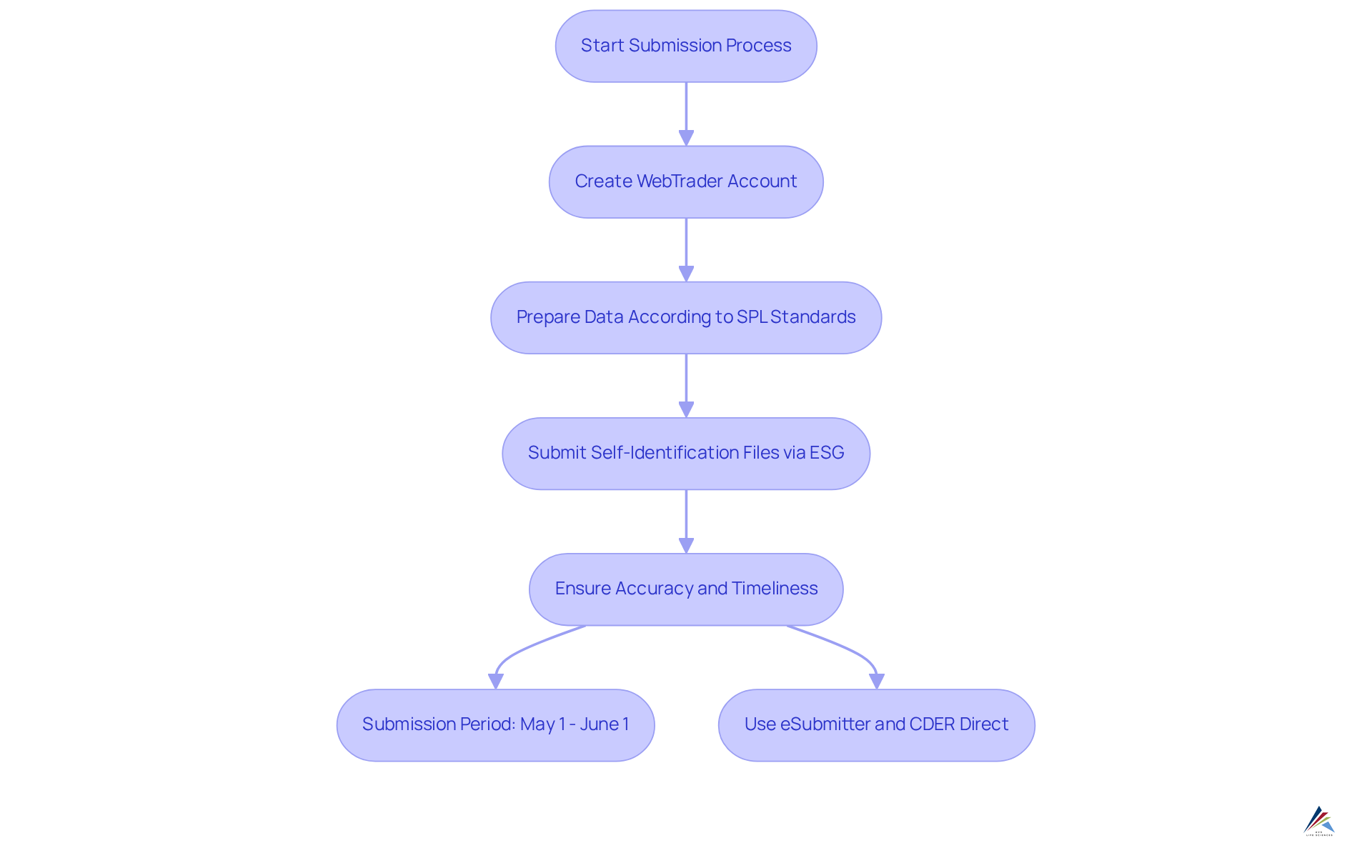
Submission Process: How to Submit GDUFA Self-Identification Information
To effectively submit information for gdufa self identification related to user fees, establishments must utilize the FDA's (ESG). A WebTrader account with the FDA ESG is required to submit self-identification files. The submission period is strictly defined, opening annually from May 1 to June 1. During this period, it is essential for establishments to prepare their data in accordance with Structured Product Labeling (SPL) standards, necessitating the inclusion of critical details such as D-U-N-S numbers and identifiers.
Accuracy in data submission is paramount; any discrepancies or late submissions can lead to significant penalties, including potential prosecution for misbranding violations.
Employing tools such as eSubmitter, a free application for generating, validating, and submitting gdufa self identification data, and CDER Direct can simplify the process, ensuring adherence to regulatory standards while promoting efficient data management.
It is vital for organizations to prioritize these steps to uphold compliance and evade the consequences of erroneous submissions.
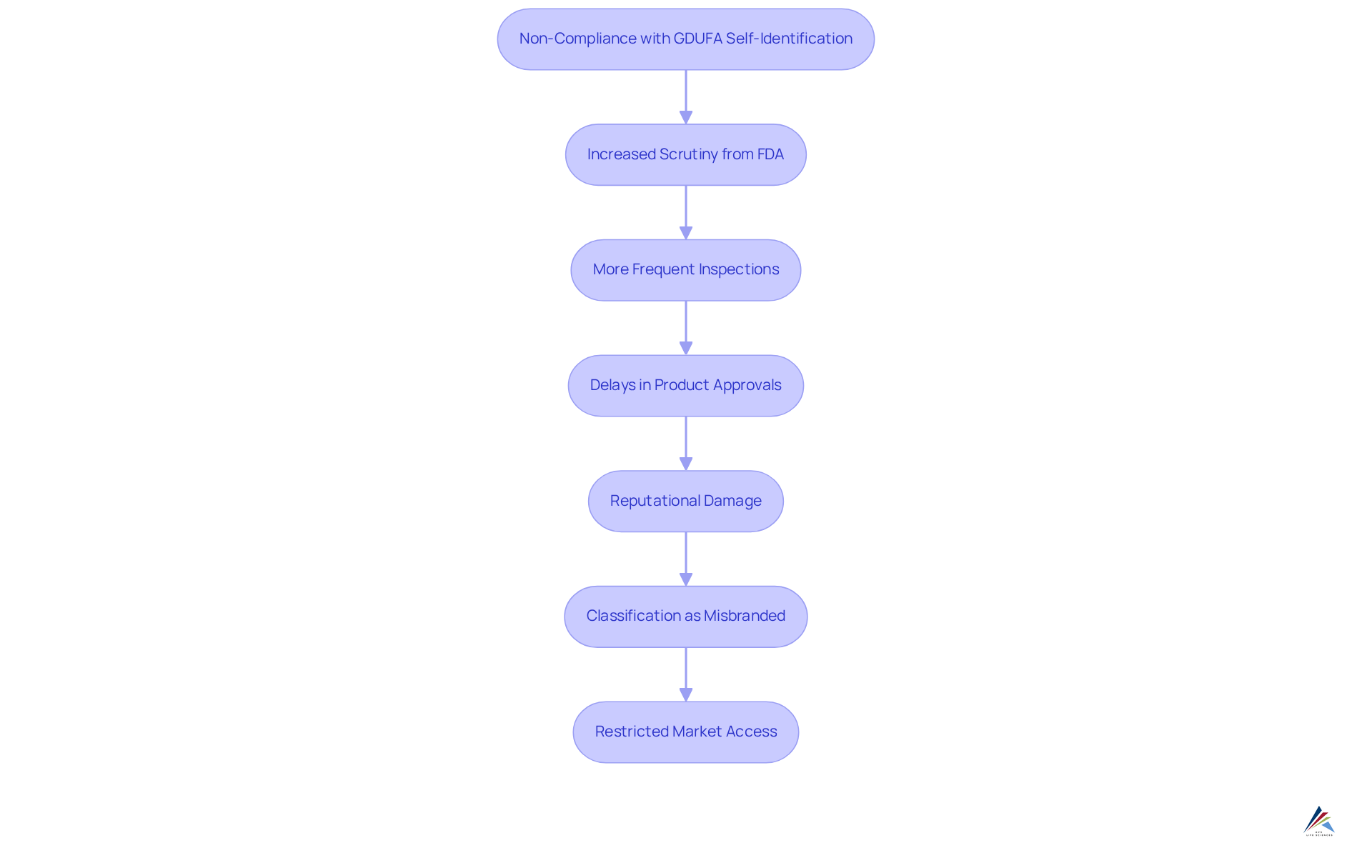
Consequences of Non-Compliance: Penalties for Failing to Self-Identify
Not self-identifying under the regulatory framework of GDUFA self-identification can have serious consequences for organizations, particularly regarding their ability to promote products manufactured at non-compliant sites. While GDUFA does not outline specific , the repercussions can be significant. Facilities that overlook this requirement may encounter increased scrutiny from the FDA, resulting in more frequent inspections and potential delays in product approvals. Such scrutiny can also lead to reputational damage, as companies may be viewed as non-compliant or unreliable by consumers and partners alike.
Moreover, items produced at locations that fail to self-identify risk being classified as misbranded, which can obstruct their importation into the United States. This predicament not only restricts market access but can also incur considerable financial losses and legal challenges. According to the FDA, the failure to comply with GDUFA self-identification renders all FDF or API products manufactured at the facility misbranded. Organizations must prioritize adherence to regulatory guidelines to mitigate these risks and ensure a smooth path to market for their products. As emphasized by Ali Gallagher, engaging a knowledgeable FDA consulting partner with legal expertise is critical to your organization’s success, underscoring the vital importance of compliance to avoid severe repercussions.
Understanding Fees: What Costs Are Involved in GDUFA Self-Identification?
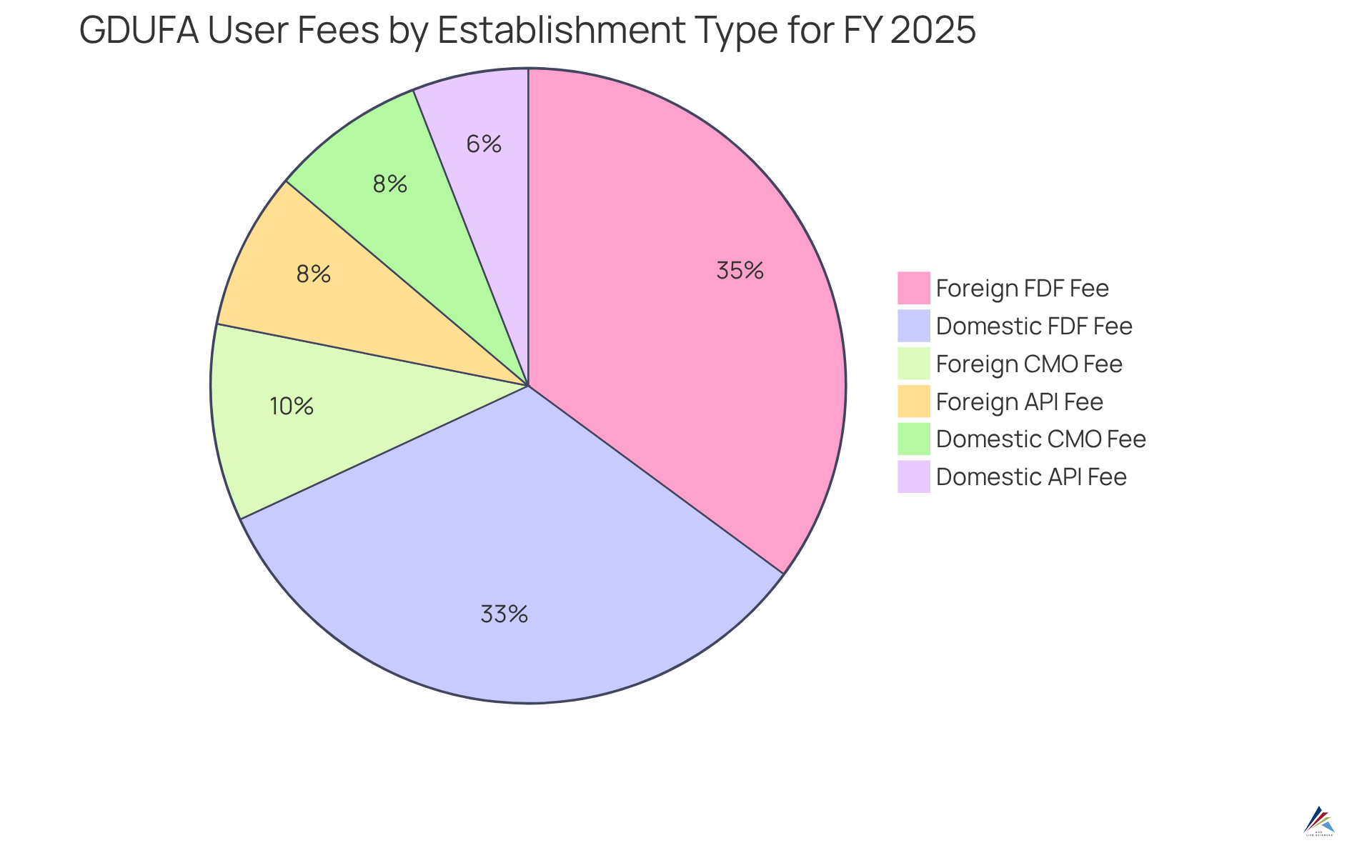
Establishments that go through GDUFA self identification are subject to user fees, with costs varying significantly based on the type of establishment and the products manufactured. For instance:
- Domestic API establishments incur an annual charge of $41,580.
- Foreign API sites face a higher cost of $56,580 due to increased inspection expenses.
- Domestic CMO locations are charged $55,668.
- Foreign CMO sites incur a fee of $70,668.
- Establishments producing Finished Dosage Forms (FDFs) are subject to different fee structures, with domestic FDF establishments charged $231,952 and foreign ones $246,952.
Organizations must strategically allocate funds for these fees as part of their regulatory efforts, ensuring they are financially prepared for yearly submissions. This proactive approach is essential for upholding regulations and avoiding penalties, as can result in significant operational setbacks, including the inability to submit new ANDAs.
The overall revenue figure for FY 2025 is estimated to reach $638,962,000, with fees from the program making a substantial contribution to this total. Grasping these expenses is crucial for efficient financial planning in relation to regulatory requirements. As financial expert Lauren K. Roth pointed out, "Budgeting for compliance with regulations is not merely about fulfilling legal obligations; it's about guaranteeing the sustainability of your operations in a competitive market.
Documentation Management: Best Practices for GDUFA Self-Identification
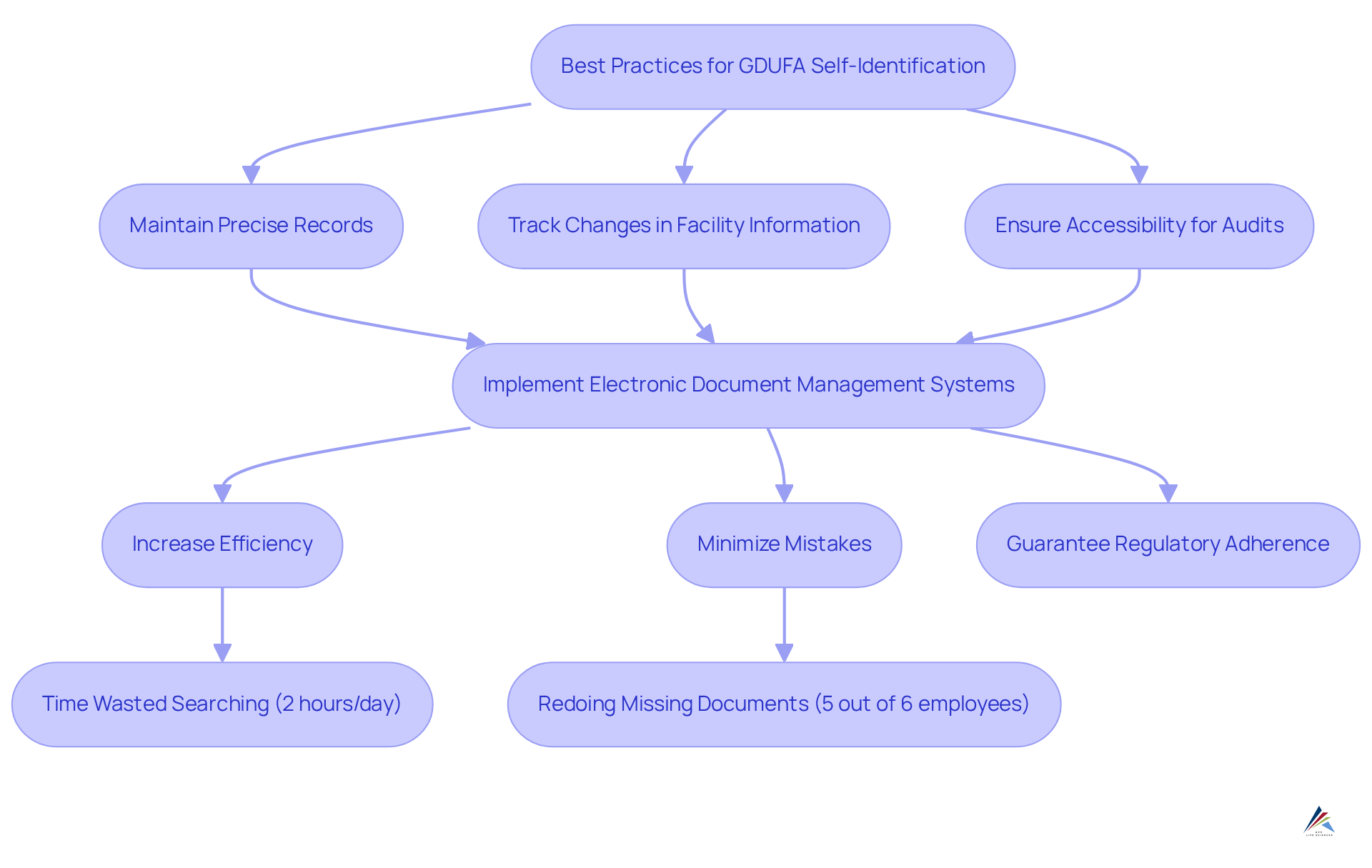
To attain effective gdufa self identification under the relevant guidelines, organizations must implement thorough . This involves:
- Maintaining precise records of all submissions
- Diligently tracking changes in facility information
- Ensuring that all documentation is easily accessible for audits
The implementation of electronic document management systems is crucial, as they can significantly streamline these processes. In fact, only 28% of small and medium-sized businesses are in advanced stages of digitization, and only 3% of companies surveyed have robust document management processes, highlighting a critical area for improvement. By employing these systems, companies can improve their efficiency, minimize the risk of mistakes, and guarantee adherence to regulatory requirements.
Inefficient document management can lead to significant time wasted, with employees spending an average of two hours per day searching for documents. Furthermore, five out of six employees have had to redo missing documents, underscoring the importance of effective document management systems. Efficient documentation approaches not only enable smoother audits but also enhance overall operational excellence, as companies can swiftly access required information when necessary. As the landscape of regulatory adherence evolves, investing in strong electronic document management solutions becomes increasingly vital for maintaining relevant standards.
Audit Preparedness: Ensuring Compliance with GDUFA Self-Identification
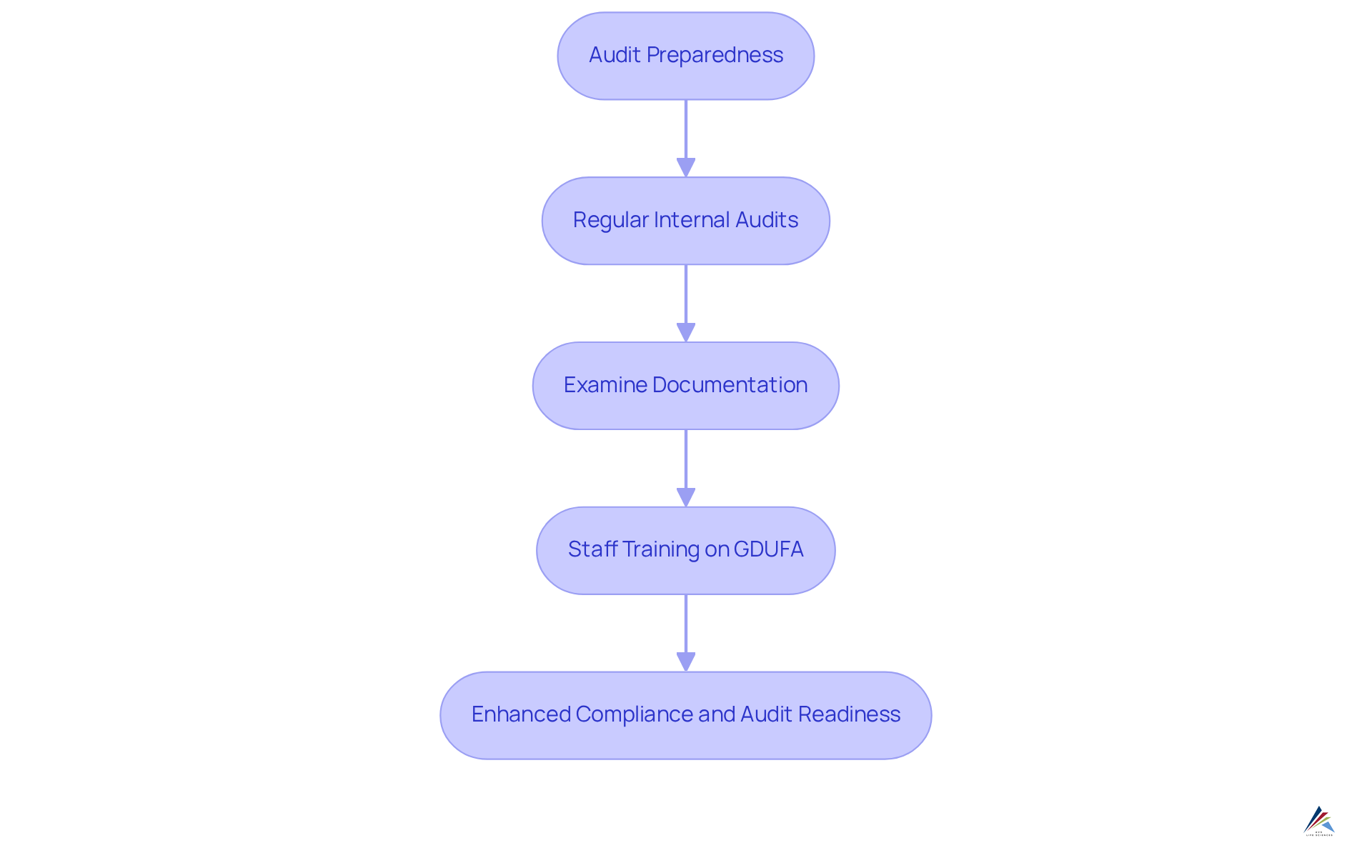
To effectively showcase adherence to self-identification requirements, organizations must prioritize audit readiness. This involves:
- Performing regular internal audits to assess adherence status.
- Carefully examining documentation for accuracy.
- Ensuring that all staff are well-trained on GDUFA self-identification.
A systematic internal audit procedure not only uncovers possible adherence gaps but also promotes a culture of responsibility and ongoing enhancement. By incorporating adherence into the organizational ethos, companies can significantly enhance their readiness for external audits and inspections, ultimately leading to smoother interactions with regulatory bodies.
Training programs that highlight the significance of can empower employees, ensuring they grasp their responsibilities in upholding standards and aiding the entity's overall audit preparedness. Moreover, with 70% of corporate risk and regulatory professionals transitioning to a more strategic approach, it is essential for companies to implement thorough audit readiness plans. As Jim Dawson states, "To be audit-ready, certain key elements must be in place."
Leveraging technology can also streamline the audit process, making it more efficient and effective. This strategic approach not only fosters compliance but also positions organizations for success in navigating the complexities of regulatory environments.
Training and Resources: Staying Informed on GDUFA Self-Identification
To ensure compliance with GDUFA self-identification requirements, organizations must prioritize ongoing training for their staff. This includes active participation in workshops, webinars, and industry conferences that focus on regulatory adherence. Notably, approximately 60% of regulatory officers engage in such events to enhance their knowledge and skills, as highlighted by Globalscape and the Ponemon Institute.
Upcoming workshops, particularly those organized by the FDA and industry experts, will provide critical insights into and adherence strategies. Moreover, utilizing resources such as the FDA's guidance documents and reputable industry publications is essential for remaining informed about regulatory changes and best practices.
As Ayush Saxena, a senior security and adherence writer, asserts, "Organizations are rapidly adopting automation technologies for regulations, creating cybersecurity awareness among employees, and investing towards a better cybersecurity framework," which is vital for effectively navigating the evolving regulatory landscape.
Leveraging Technology: Tools to Facilitate GDUFA Self-Identification
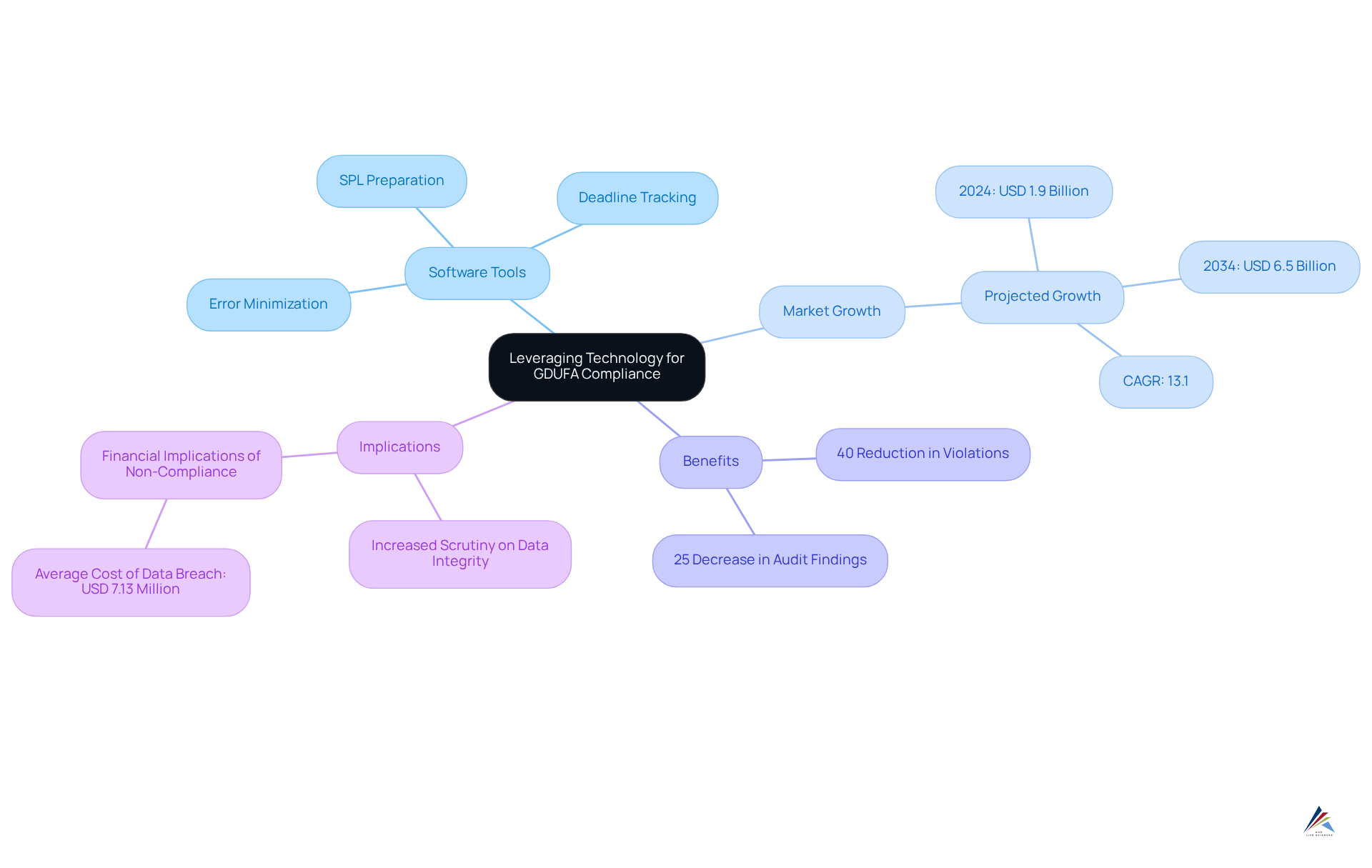
Organizations can significantly enhance their adherence to GDUFA self-identification by leveraging specialized software tools tailored for regulatory submissions. These tools not only but also effectively track submission deadlines and manage essential documentation. The integration of automated systems minimizes the risk of errors and ensures timely submissions, thus bolstering overall adherence efforts.
Given that the pharmaceutical quality management software market is projected to grow from USD 1.9 billion in 2024 to USD 6.5 billion by 2034, at a compound annual growth rate (CAGR) of 13.1%, the adoption of such technologies is becoming increasingly critical. Furthermore, firms utilizing predictive analytics report a 40% reduction in regulatory violations and a 25% decrease in audit findings, underscoring the transformative impact of automation on submission accuracy and timeliness.
As regulatory bodies intensify their scrutiny on data integrity, the implementation of these advanced tools is anticipated to become standard practice within the industry. Additionally, with the average cost of a data breach in healthcare reaching USD 7.13 million, the financial implications of non-compliance further highlight the necessity of employing advanced regulatory tools.
Industry Impact: The Significance of GDUFA Self-Identification for Compliance Officers
For regulatory officers, GDUFA self-identification is not just important; it is crucial. It directly influences an entity's capacity to promote generic drugs and shapes overall adherence strategies. This procedure guarantees conformity to regulatory standards and enables oversight personnel to promote essential resources and training within their entities.
As the regulatory environment evolves, understanding the importance of self-identification is essential for upholding standards and mitigating risks. Data indicates that:
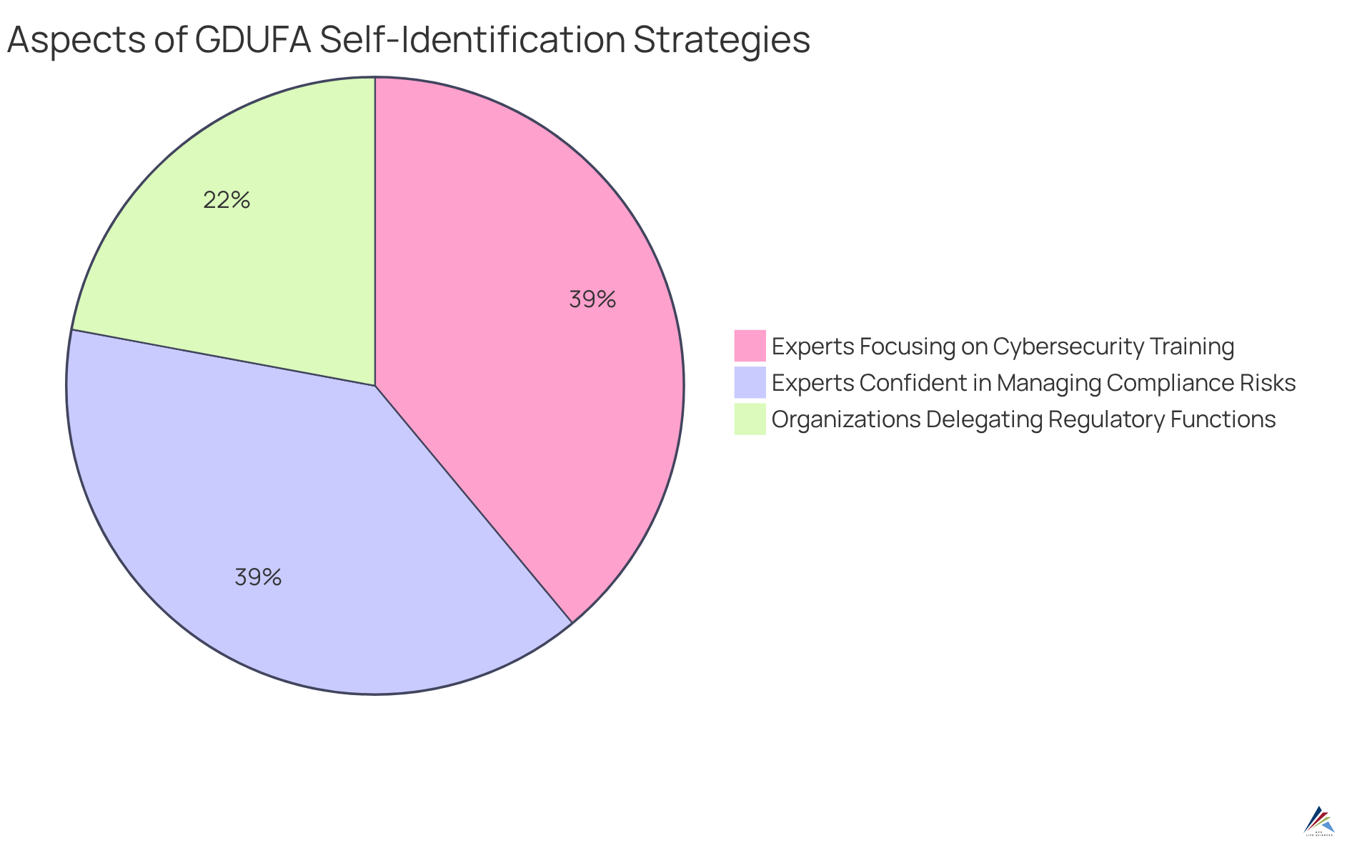
- 34% of organizations delegate certain regulatory functions to address complex requirements, underscoring the necessity for adequate internal resources to support regulatory adherence.
- 60% of risk and regulatory experts plan to focus on cybersecurity training in the next two to three years, signaling a strategic shift in oversight management that aligns with GDUFA standards.
- Three out of five corporate risk and regulatory experts express confidence in , highlighting the significance of GDUFA self-identification in enhancing regulatory strategies.
By aligning compliance strategies with GDUFA requirements, organizations can not only enhance their operational efficiency but also ensure they remain competitive in the market.
Conclusion
Navigating the intricacies of GDUFA self-identification compliance is essential for organizations involved in the production of generic drugs. This process not only ensures adherence to regulatory standards but also protects market access and operational integrity. The steps for compliance encompass understanding the self-identification requirements and leveraging technology for accurate submissions, all of which are critical in maintaining a competitive edge in the pharmaceutical landscape.
Key aspects include:
- The necessity of thorough documentation management
- The implications of non-compliance
- The importance of training and resources
Establishments must recognize the potential risks associated with failing to self-identify, including increased scrutiny from regulatory bodies and financial penalties. By employing effective compliance strategies and engaging with experienced partners like AVS Life Sciences, organizations can navigate these challenges successfully.
Ultimately, the significance of GDUFA self-identification extends beyond mere compliance; it is a foundational element for fostering trust and reliability within the pharmaceutical industry. As regulatory landscapes evolve, organizations are encouraged to prioritize ongoing education and invest in technology solutions that streamline compliance processes. By doing so, they not only enhance their operational efficiency but also contribute to a more robust and transparent industry, ensuring that they remain well-positioned for future challenges.
Frequently Asked Questions
What services does AVS Life Sciences provide for GDUFA compliance?
AVS Life Sciences offers a comprehensive suite of services including validation, quality assurance consulting, and engineering assistance to help organizations navigate the complexities of GDUFA self-identification.
Why is regulatory compliance important for organizations in the pharmaceutical sector?
Regulatory compliance is crucial for maintaining a competitive advantage and securing market access, as it ensures that organizations meet rigorous quality standards throughout the product lifecycle.
Who is required to comply with GDUFA self-identification?
All establishments involved in the production of human generic medications, including Active Pharmaceutical Ingredients (APIs) and Finished Dosage Forms (FDFs), must complete GDUFA self-identification with the FDA, regardless of their domestic or international location.
What are the consequences of failing to self-identify under GDUFA?
Failure to self-identify can lead to serious repercussions, including the potential misbranding of all products produced at the site, making compliance critical for all stakeholders in the generic drug manufacturing landscape.
How many establishments must comply with GDUFA self-identification?
There are 153 domestic FDF establishments and 325 foreign FDF entities that must adhere to GDUFA self-identification requirements.
What is the submission process for GDUFA self-identification information?
Establishments must use the FDA's Electronic Submission Gateway (ESG) and have a WebTrader account to submit self-identification files. The submission period is from May 1 to June 1 each year.
What standards must be followed when submitting GDUFA self-identification data?
Submissions must comply with Structured Product Labeling (SPL) standards and include critical details such as D-U-N-S numbers and identifiers.
What tools can assist in the GDUFA self-identification submission process?
Tools such as eSubmitter, a free application for generating, validating, and submitting GDUFA self-identification data, and CDER Direct can simplify the submission process and ensure regulatory compliance.
What can happen if there are discrepancies or late submissions of GDUFA self-identification data?
Discrepancies or late submissions can lead to significant penalties, including potential prosecution for misbranding violations.
