10 Essential Skills for Drug Safety Jobs in 2025

Overview
The primary objective of this article is to delineate the essential skills necessary for drug safety positions in 2025. It is imperative for professionals in this domain to exhibit competencies such as:
- Regulatory compliance knowledge
- Risk management
- Data analysis
- Effective communication
- Attention to detail
- Project management
- Commitment to continuous learning
These skills are not merely advantageous; they are critical for ensuring drug safety and adeptly navigating the ever-evolving pharmaceutical landscape. As the industry progresses, the demand for such expertise will only intensify, underscoring the necessity for ongoing professional development and adaptability.
Introduction
The landscape of drug safety is undergoing a significant transformation, propelled by escalating regulatory demands and the imperative for enhanced patient protection. With the global market for drug safety monitoring poised for substantial growth, professionals in this arena must cultivate a diverse skill set to adeptly navigate the complexities of pharmacovigilance.
What essential skills will delineate success in drug safety roles by 2025? How can professionals position themselves advantageously in this dynamic environment? This article explores ten critical competencies that will define the future of drug safety positions, ensuring that practitioners are thoroughly prepared to tackle the challenges and seize the opportunities that lie ahead.
AVS Life Sciences: Expertise in Drug Safety and Pharmacovigilance
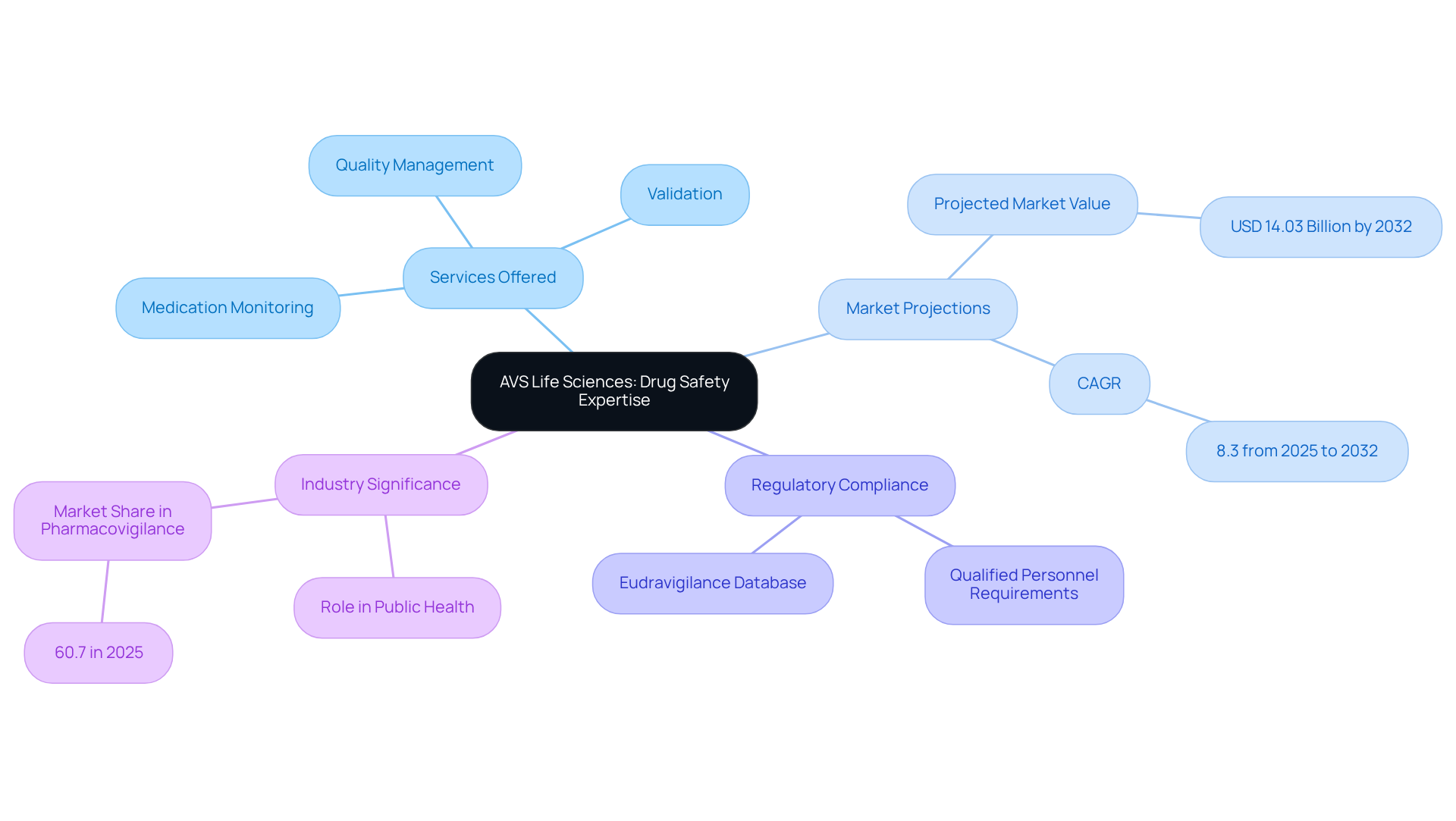
AVS Life Sciences stands at the forefront of drug safety jobs and medication monitoring services tailored for the pharmaceutical and biotechnology sectors. Their comprehensive offerings include validation, regulatory compliance, and quality management—essential components for navigating the intricate regulatory landscapes governing pharmaceutical development.
With a dedicated team of over 300 seasoned professionals, AVS Life Sciences is unwavering in its commitment to maintaining the highest standards of quality and compliance throughout the entire lifecycle of medicinal products. This dedication is particularly crucial as the global drug safety monitoring market is projected to reach USD 14.03 billion by 2032, reflecting a compound annual growth rate (CAGR) of 8.3% from 2025 to 2032.
The firm’s expertise not only ensures adherence to stringent regulations but also positions them as a trusted partner for organizations aiming to implement effective medication monitoring programs. As oversight intensifies, the significance of drug safety jobs in monitoring drug safety escalates, underscoring the vital role AVS Life Sciences plays in safeguarding public health and safety.
Moreover, with national competent authorities and the European Medicines Agency (EMA) mandating qualified personnel, AVS Life Sciences is exceptionally equipped to fulfill these requirements. Their extensive industry expertise, which includes GMP audits across API and drug product CMOs, contract testing laboratories, and manufacturing locations, fortifies their strategic positioning in the drug safety jobs market, projected to hold the largest share of 60.7% in 2025.
Additionally, the Eudravigilance database is instrumental in disseminating drug safety information among competent authorities, further emphasizing the significance of AVS's services in ensuring regulatory compliance. Monitoring unique circumstances, such as pregnancy and pediatric groups, is also a critical element of effective drug safety practices that AVS Life Sciences adeptly addresses.
As the market evolves, the responsibilities of marketing authorization holders (MAHs) in pharmacovigilance will continue to shape the landscape, making AVS's expertise indispensable.
Regulatory Compliance: Mastering Industry Standards and Guidelines
A thorough understanding of compliance with regulations is essential for professionals in drug safety jobs, particularly concerning Good Manufacturing Practices (GMP), International Organization for Standardization (ISO) standards, and Quality System Regulations (QSR). Mastery of these frameworks is crucial for ensuring that all drug safety jobs adhere to legal and ethical standards, ultimately protecting public health. Continuous education and training in drug safety jobs are imperative as they enable professionals to stay abreast of evolving regulations and maintain compliance effectively.
The pharmaceutical industry faces significant compliance challenges, which underscores the need for drug safety jobs, as evidenced by the FDA issuing 3,344 observations for noncompliance in 2018 alone. Firms that excel in mastering GMP and ISO standards, such as AVS Life Sciences, exemplify the importance of robust quality management systems for drug safety jobs that not only meet compliance requirements but also enhance operational efficiency. AVS Life Sciences provides extensive consulting services that assist organizations in navigating the complexities of compliance and quality management related to drug safety jobs, ensuring adherence to the highest standards.
As policy modifications continue to influence the landscape of drug safety jobs in 2025, experts must adapt to new benchmarks and methodologies. The adoption rates of ISO standards in pharmaceutical quality are steadily increasing, reflecting a growing acknowledgment of their significance in ensuring product quality and safety. By fostering a culture of adherence and continuous improvement, organizations can more effectively manage the complexities of regulatory landscapes and ensure the quality and effectiveness of drug safety jobs.

Risk Management: Identifying and Mitigating Safety Concerns
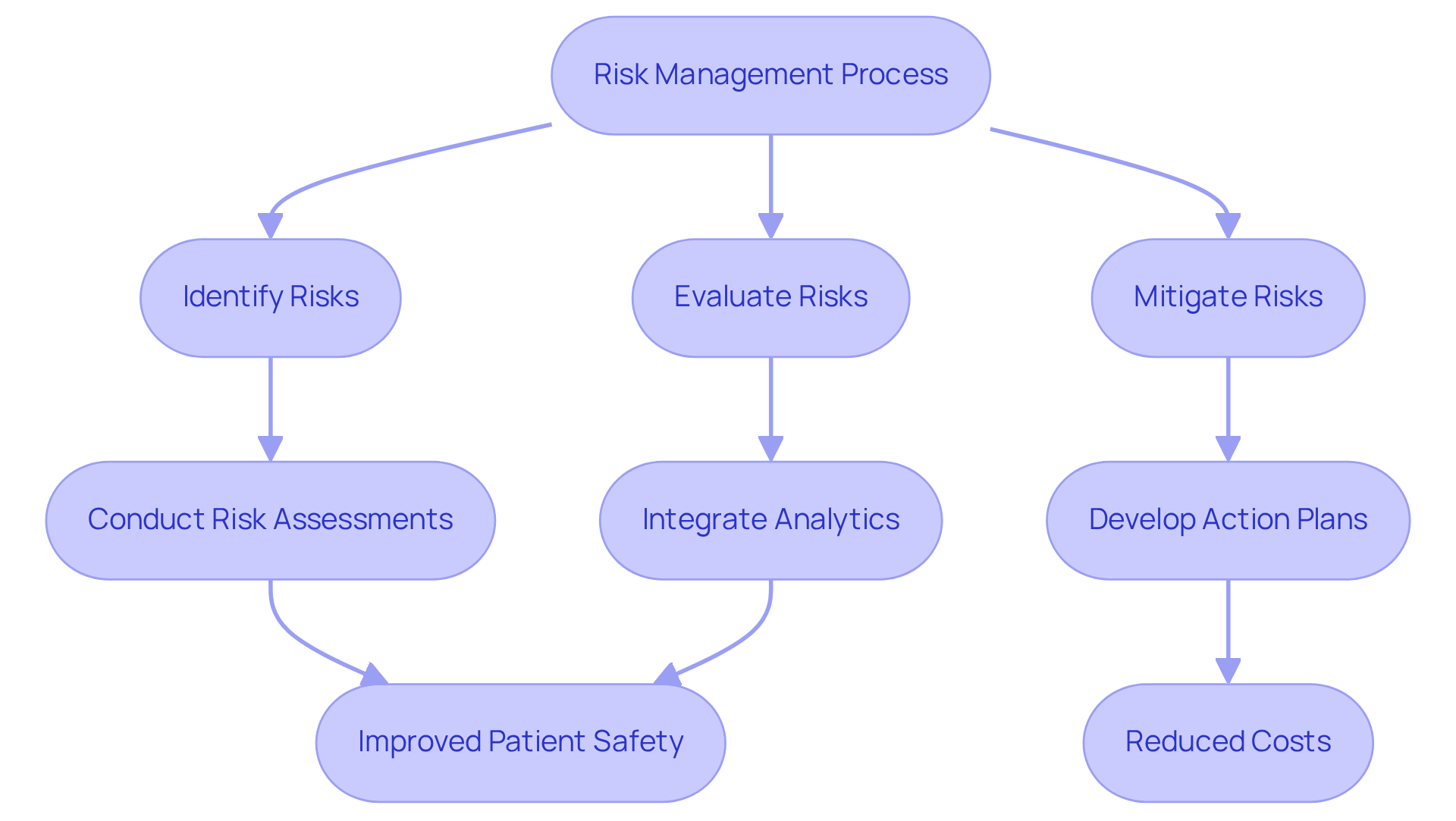
Risk management in pharmaceuticals represents a systematic approach that involves identifying, evaluating, and mitigating potential health risks associated with medicinal products. Professionals in this field must excel in conducting thorough risk assessments, which are vital for recognizing adverse impacts and safeguarding patient well-being throughout the drug lifecycle. Effective risk management plans are essential; they outline specific actions to address identified risks and integrate proactive strategies to minimize adverse effects.
Recent trends indicate a significant shift towards advanced analytics in risk management. For instance, the application of risk analysis has led to an impressive 86% reduction in negative event reporting durations, decreasing from an average of 16.2 days to just 2.3 days. Moreover, healthcare systems that have adopted extensive analytics programs have experienced a 23% reduction in costs associated with preventable adverse events, resulting in a remarkable 430% return on investment.
Case studies from 2025 underscore the effectiveness of structured risk management plans. A notable example is a comprehensive study conducted across 42 hospitals, which reported a 42% reduction in medication errors, preventing approximately 1.2 million errors annually. This illustrates the importance of integrating real-time analytics and fostering a culture that prioritizes patient well-being.
As the pharmaceutical landscape continues to evolve, staying informed about these trends and employing robust risk management strategies will be imperative for professionals aiming to enhance drug security and efficacy. AVS Life Sciences provides comprehensive quality management and regulatory compliance solutions that can bolster these initiatives, particularly in overcoming challenges such as data integration across disparate systems. By leveraging AVS Life Sciences' expertise, professionals can fortify their risk management strategies and ultimately improve patient well-being.
Data Analysis: Interpreting Safety Data for Informed Decisions
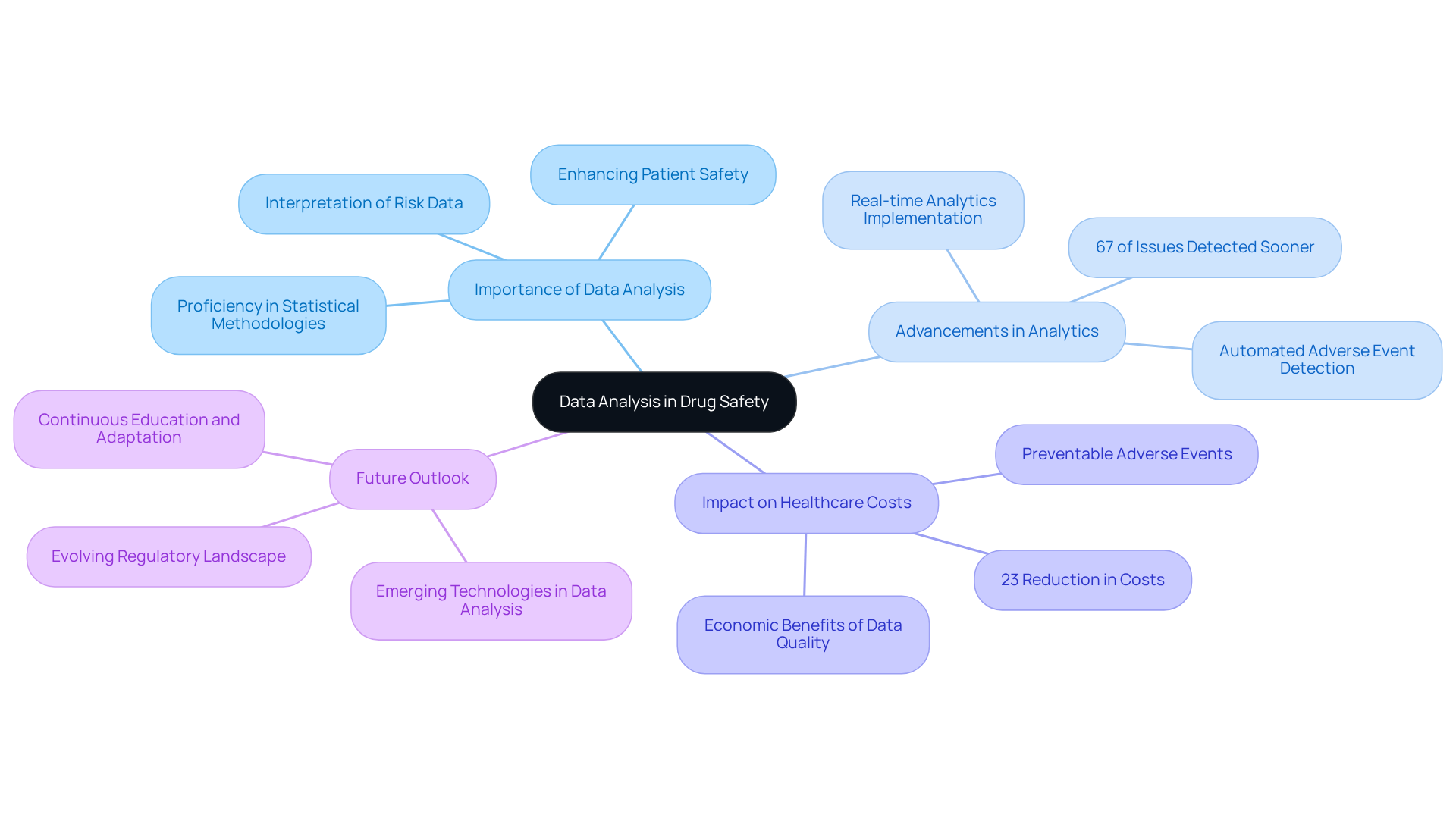
Data analysis stands as a critical competency for drug safety jobs, enabling precise interpretation of risk data. This involves a thorough examination of adverse event reports, identifying patterns, and evaluating the comprehensive risk profile of pharmaceuticals. Proficiency in statistical methodologies and data management tools is essential for extracting actionable insights from intricate datasets, ultimately guiding compliance decisions and enhancing patient safety.
Recent advancements in data analysis tools for risk management have significantly bolstered the ability to detect signals earlier. Studies reveal that 67% of serious issues could be identified an average of 18 months sooner using advanced analytics. This proactive approach not only facilitates prompt regulatory responses but also cultivates a culture of well-being within healthcare organizations, benefiting professionals in drug safety jobs. Moreover, entities that emphasize data quality can realize a 23% reduction in healthcare costs linked to preventable adverse events.
As Albert Einstein astutely noted, "Concern for humanity and his well-being must always constitute the primary focus of all technical efforts." This sentiment resonates deeply within the realm of drug safety jobs, where health data analysis is vital for safeguarding public health. Additionally, as Terry J. Moulton articulated, "A culture of security is a journey, not a destination," underscoring the ongoing commitment required in pharmacovigilance and data protection practices.
Looking ahead to 2025, the landscape of pharmaceutical oversight will continue to evolve, with an increasing emphasis on leveraging advanced data analysis tools to refine decision-making processes. As specialists in the field advocate for continuous education and adaptation to emerging technologies, the ability to analyze health data effectively will remain a cornerstone of successful drug safety jobs. To maintain a competitive edge, professionals should engage in regular training on the latest data management tools and methodologies.
Communication Skills: Collaborating Across Teams and Stakeholders
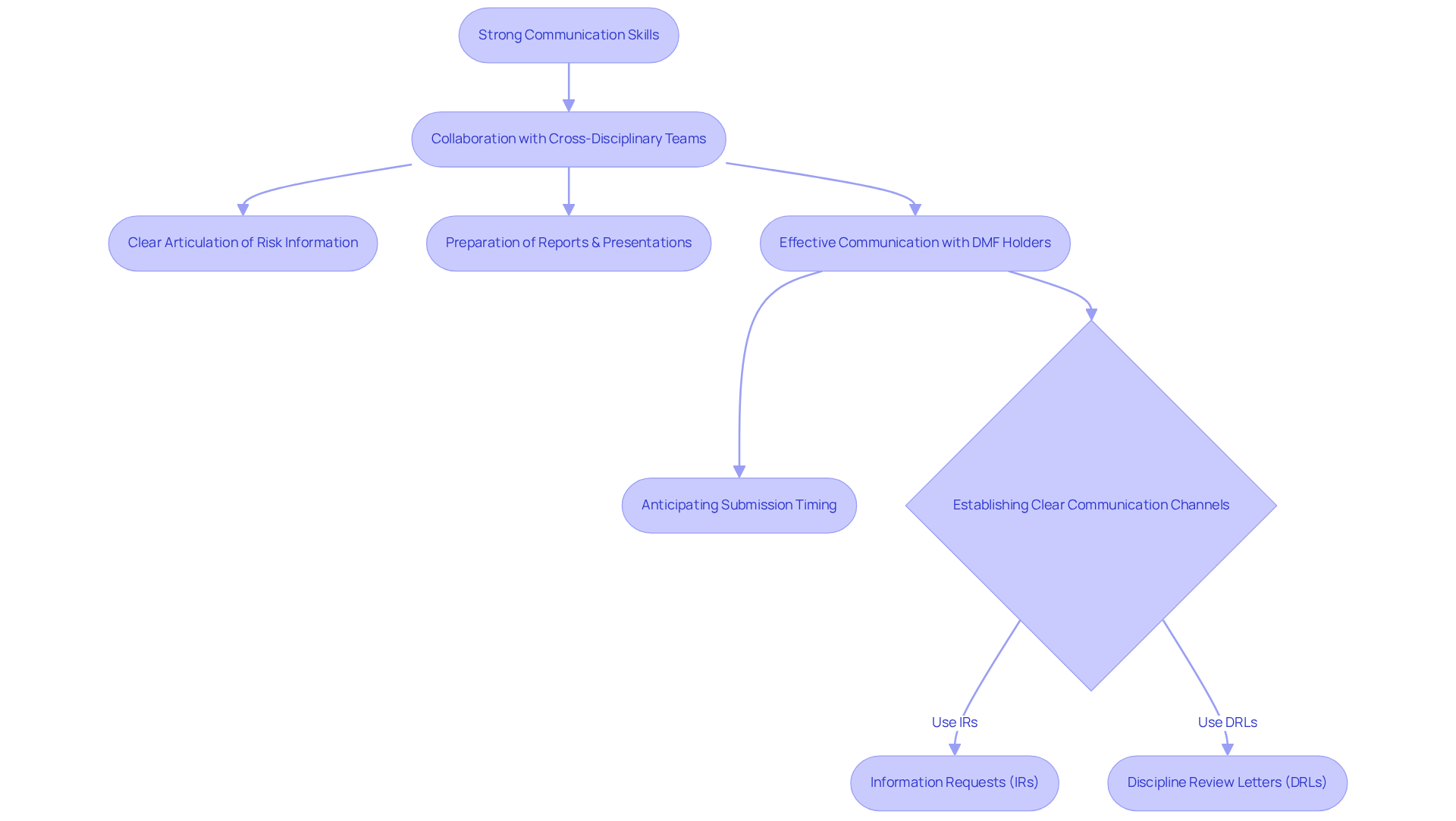
Strong communication abilities are essential for pharmaceutical professionals, who frequently collaborate with cross-disciplinary teams, regulatory bodies, and healthcare providers. The capacity to articulate intricate risk information clearly and concisely is vital for ensuring that all stakeholders comprehend potential hazards and precautionary measures. This encompasses the preparation of comprehensive reports, the delivery of informative presentations, and participation in discussions that foster transparency and collaboration in pharmaceutical initiatives.
In 2025, the significance of collaboration in pharmacovigilance is underscored by the necessity for timely communication among team members and stakeholders. For instance, effective communication with Drug Master File (DMF) holders is crucial to anticipate submission timing, as delays can adversely affect ANDA goal dates. Furthermore, establishing clear communication channels, such as Information Requests (IRs) and Discipline Review Letters (DRLs), facilitates the exchange of critical information, ensuring that necessary data is submitted promptly.
Effective medication monitoring teams emphasize cooperation, which not only enhances the quality of evaluations but also cultivates trust among team members. By promoting a culture of transparent dialogue, professionals can exchange insights and proactively address issues, ultimately leading to improved patient care outcomes. As the field of pharmacovigilance evolves, mastering effective communication skills will remain a fundamental element for success in drug safety jobs related to medication monitoring. Combining these skills with AVS Life Sciences' quality management and compliance solutions can further augment the effectiveness of pharmaceutical protection initiatives.
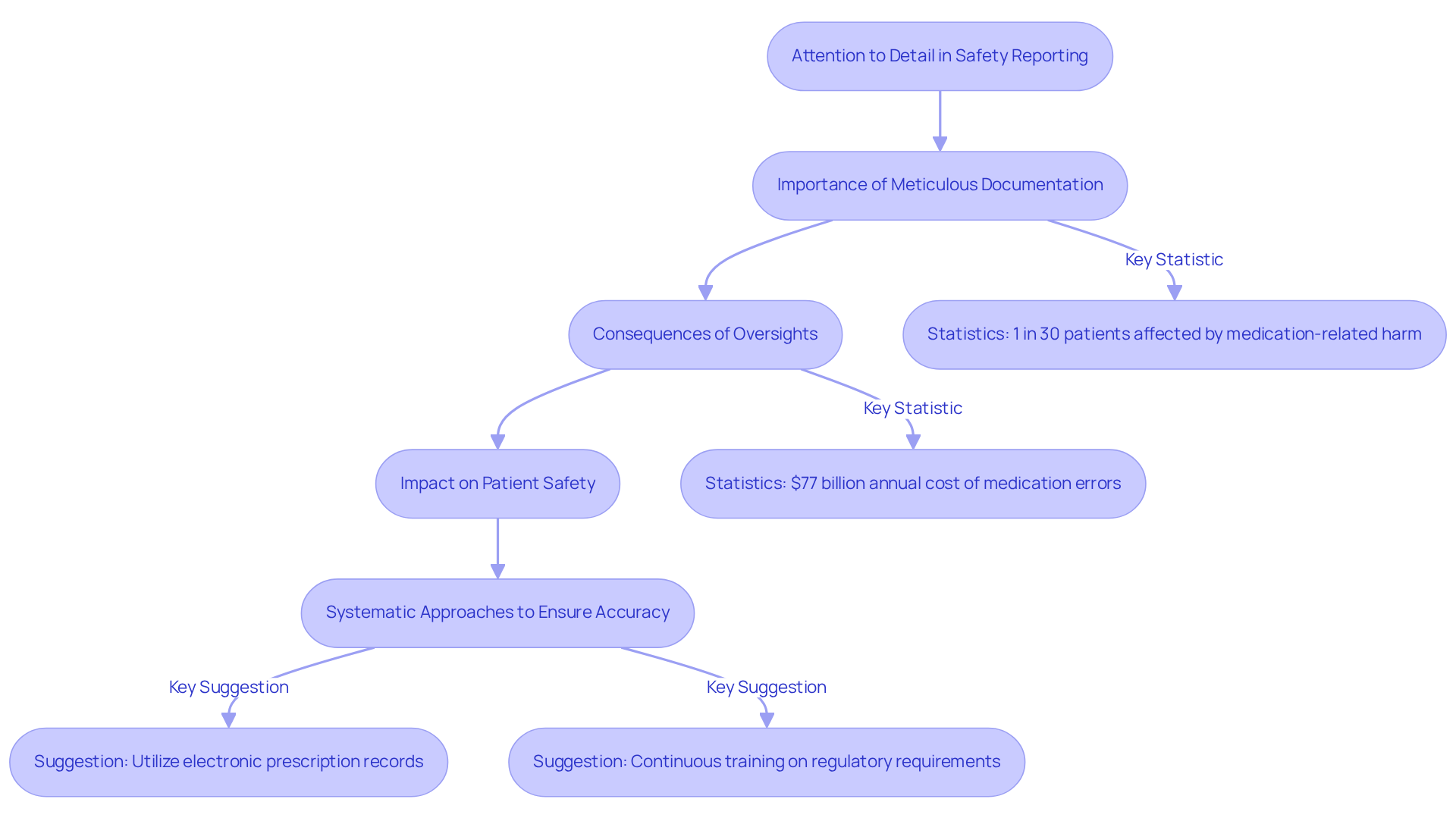
Attention to Detail: Ensuring Accuracy in Safety Reporting
Attention to detail is a crucial ability for professionals in drug safety jobs, significantly affecting the precision of reporting on risk. Meticulous documentation of adverse events is essential, as it ensures that all pertinent information is captured and reported in accordance with regulatory standards. This diligence is vital in preventing mistakes that could endanger patient well-being and uphold the integrity of the drug monitoring system.
Recent audits in 2025 have underscored the significance of precision in medication safety documentation, revealing that even minor oversights can lead to substantial consequences. For instance, medication-related harm affects 1 out of every 30 patients, with over a quarter of these cases being severe or life-threatening. Such statistics highlight the critical need for professionals to focus on precision in their reporting practices.
Experts emphasize that attention to detail not only enhances compliance but also directly impacts patient safety outcomes. A culture of thoroughness in documentation can significantly reduce the incidence of medication errors, which are estimated to cost the healthcare system $77 billion annually. By establishing strong quality control practices and promoting a culture where precision is valued, organizations can mitigate risks related to drug safety jobs.
To ensure accuracy in pharmacovigilance documentation, professionals should adopt systematic approaches, such as utilizing electronic prescription records and medication bar coding, which help safeguard against errors. Furthermore, continuous training and education on the latest regulatory requirements and best practices are crucial for maintaining high standards in reporting.
In conclusion, the commitment to detail in reporting on security is not merely a procedural necessity; it is a fundamental aspect of protecting patient health and enhancing the overall efficacy of the healthcare system.
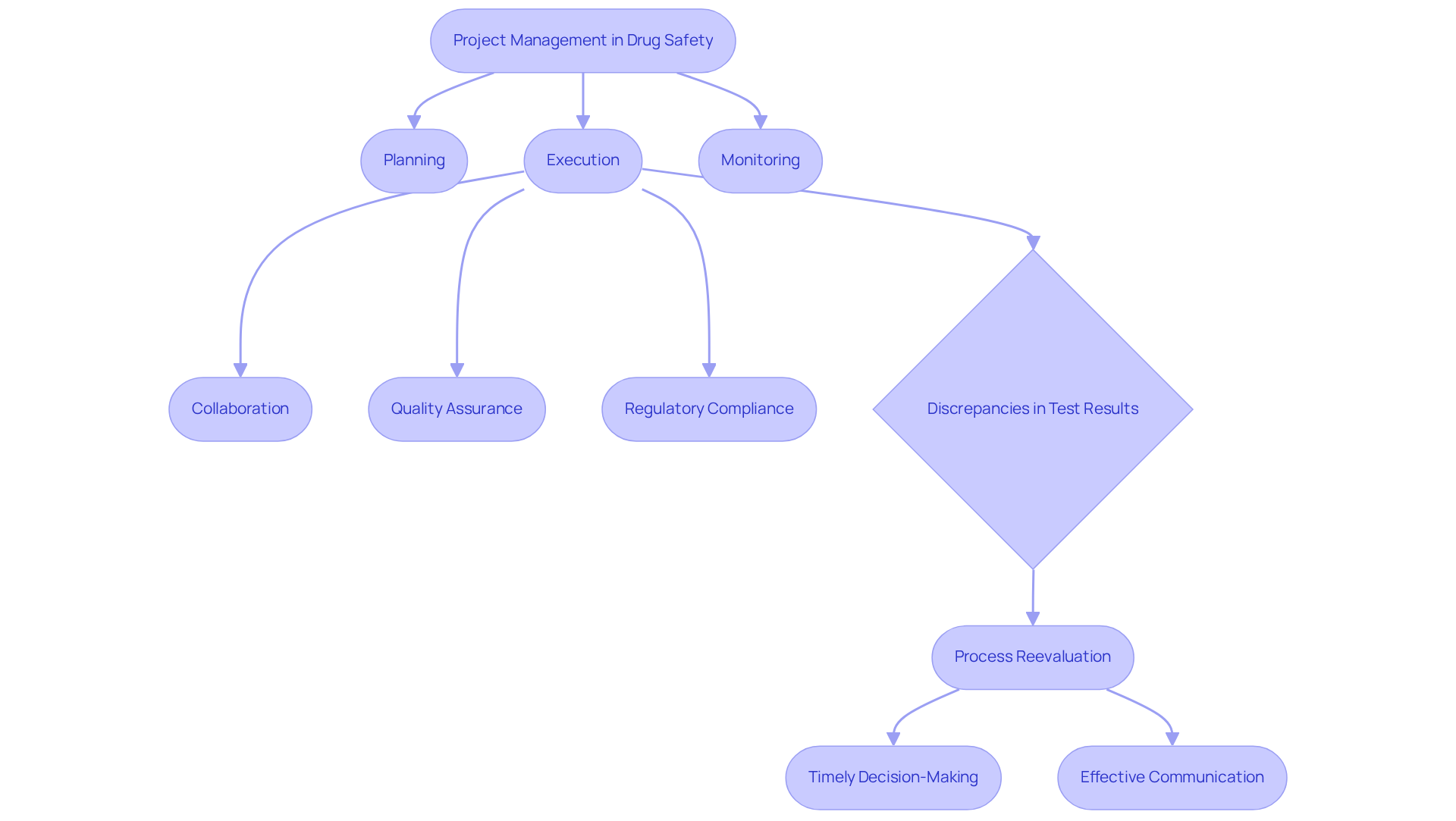
Project Management: Overseeing Drug Safety Initiatives
Project management stands as a pivotal skill for those pursuing drug safety jobs, particularly within the realm of pharmacovigilance. This discipline encompasses the planning, execution, and monitoring of safety evaluations, ensuring that all activities align with legal timelines and budget constraints. Effective project management fosters collaboration among team members, a crucial component for maintaining adherence to stringent legal requirements.
A transformative case study from AVS Life Sciences exemplifies this principle, showcasing their successful upgrade of a biotechnology GMP facility. This project adhered not only to budget and timeline but also underscored the importance of quality assurance and regulatory compliance, highlighting the critical role of comprehensive documentation practices and standard operating procedures (SOPs) in safeguarding data integrity.
During the upgrade, challenges emerged, including discrepancies in test results attributed to improperly installed barcode scanner cameras, which were initially overlooked. This experience yielded valuable lessons, prompting the QC laboratory team and Quality team to reassess their processes and enhance the reliability of test results.
As the demand for skilled project managers in drug safety jobs escalates, professionals must adeptly navigate complex regulatory landscapes and leverage advanced project management tools. Insights from industry experts emphasize that timely decision-making and effective communication are vital for the successful implementation of pharmaceutical initiatives.
As the industry evolves, the integration of innovative project management practices will be essential for bolstering the reliability and efficacy of pharmaceuticals.
Pharmacovigilance Systems: Monitoring Adverse Drug Reactions
Pharmacovigilance systems are crucial for tracking adverse medication reactions (ADRs) and ensuring the security of treatments. As we look towards 2025, advancements in these systems have led to more efficient data collection, analysis, and reporting processes. Experts must be well-versed in the various systems employed to gather, examine, and present data on safety, including electronic reporting tools and databases. Understanding how to utilize these systems effectively empowers professionals in drug safety jobs to recognize hazard signals and respond promptly to emerging issues.
Current trends indicate a significant shift towards the integration of technology in drug safety jobs, with electronic reporting systems becoming increasingly prevalent. Statistics reveal that serious ADRs accounted for 34.1% of total reports, with a mortality rate of 2.8% among serious ADRs, underscoring the necessity for robust monitoring systems. Efficient electronic reporting instruments, such as the Eudravigilance database, play a crucial role in exchanging drug monitoring information among competent authorities, ensuring that potential risk factors are communicated effectively.
Furthermore, the continuous development of drug safety monitoring systems emphasizes the importance of educating healthcare professionals about drug safety jobs and the use of these tools. By enhancing their skills in ADR monitoring, professionals can contribute to improved patient care and adherence to regulatory standards, including the submission of periodic safety update reports (PSURs). As the landscape of medication security continues to evolve, remaining informed about the latest advancements and trends in pharmacovigilance systems is essential for success in the field.

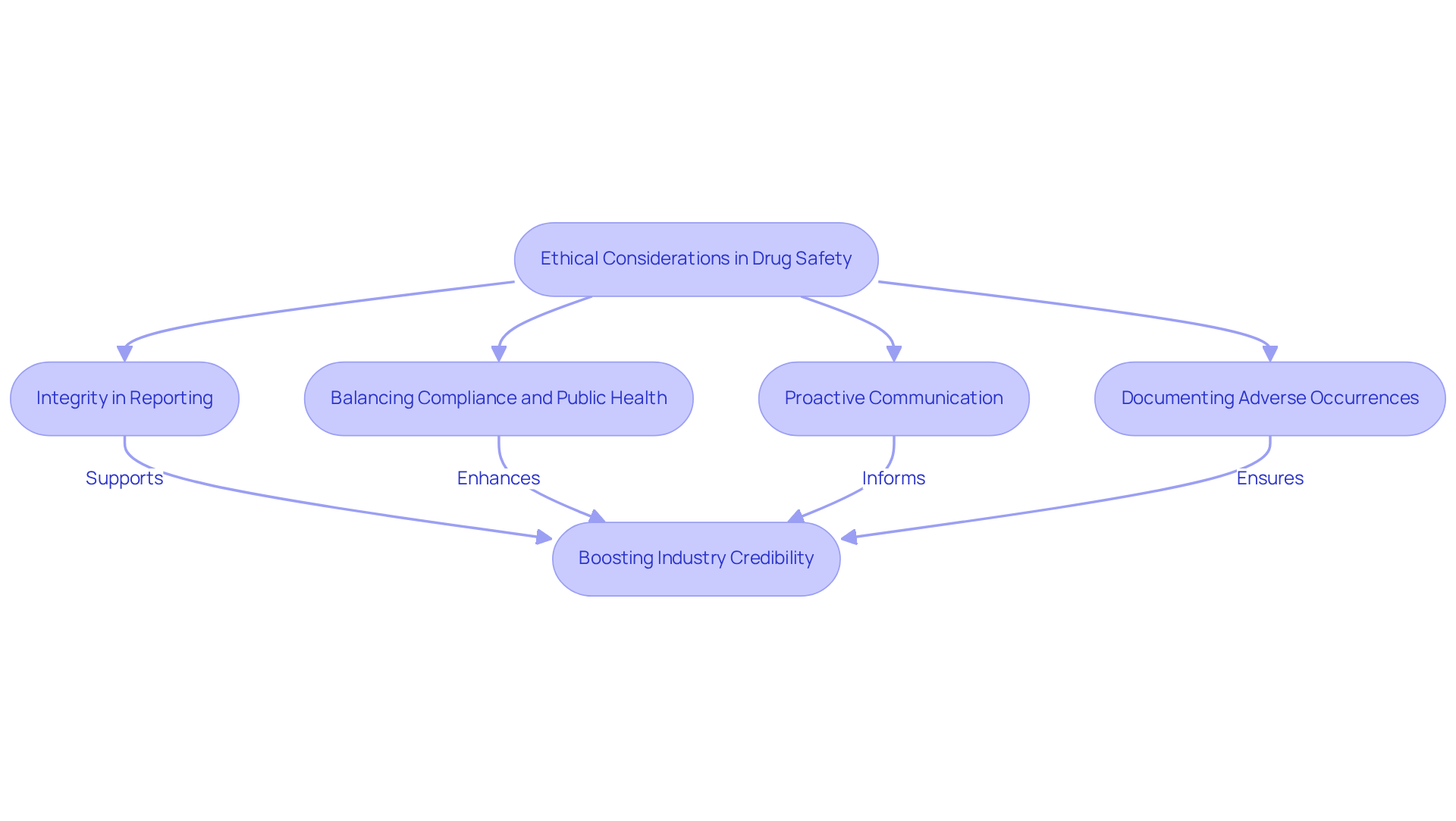
Ethical Considerations: Upholding Integrity in Drug Safety
Ethical considerations are paramount in drug protection, serving as the foundation for decision-making among professionals in the field. Maintaining integrity necessitates precise and transparent reporting of all protection data, with a firm dedication to prioritizing patient welfare. Substance security specialists confront complex ethical challenges, where they must balance compliance with the necessity to protect public health and cultivate trust in the pharmaceutical sector.
Recent ethical challenges in 2025 have highlighted the necessity for robust integrity in pharmacovigilance practices, as lapses can lead to significant repercussions for both patients and companies. Comprehensive documentation of adverse occurrences and proactive communication with regulatory agencies illustrate integrity in reporting risk data, ensuring that all stakeholders are informed and involved.
By upholding high ethical standards, pharmaceutical professionals not only safeguard patients but also boost the credibility of the industry as a whole.
Continuous Learning: Adapting to Evolving Industry Standards
Ongoing education stands as a cornerstone for pharmaceutical professionals, particularly as the industry undergoes relentless transformation driven by new regulations, technologies, and best practices. Engaging in continuous education—such as attending workshops and participating in training programs—empowers professionals in drug safety jobs to remain abreast of the latest advancements in drug protection and pharmacovigilance. This dedication not only sharpens individual expertise but also significantly contributes to drug safety jobs by ensuring the safety and efficacy of pharmaceutical products.
Notably, statistics indicate that:
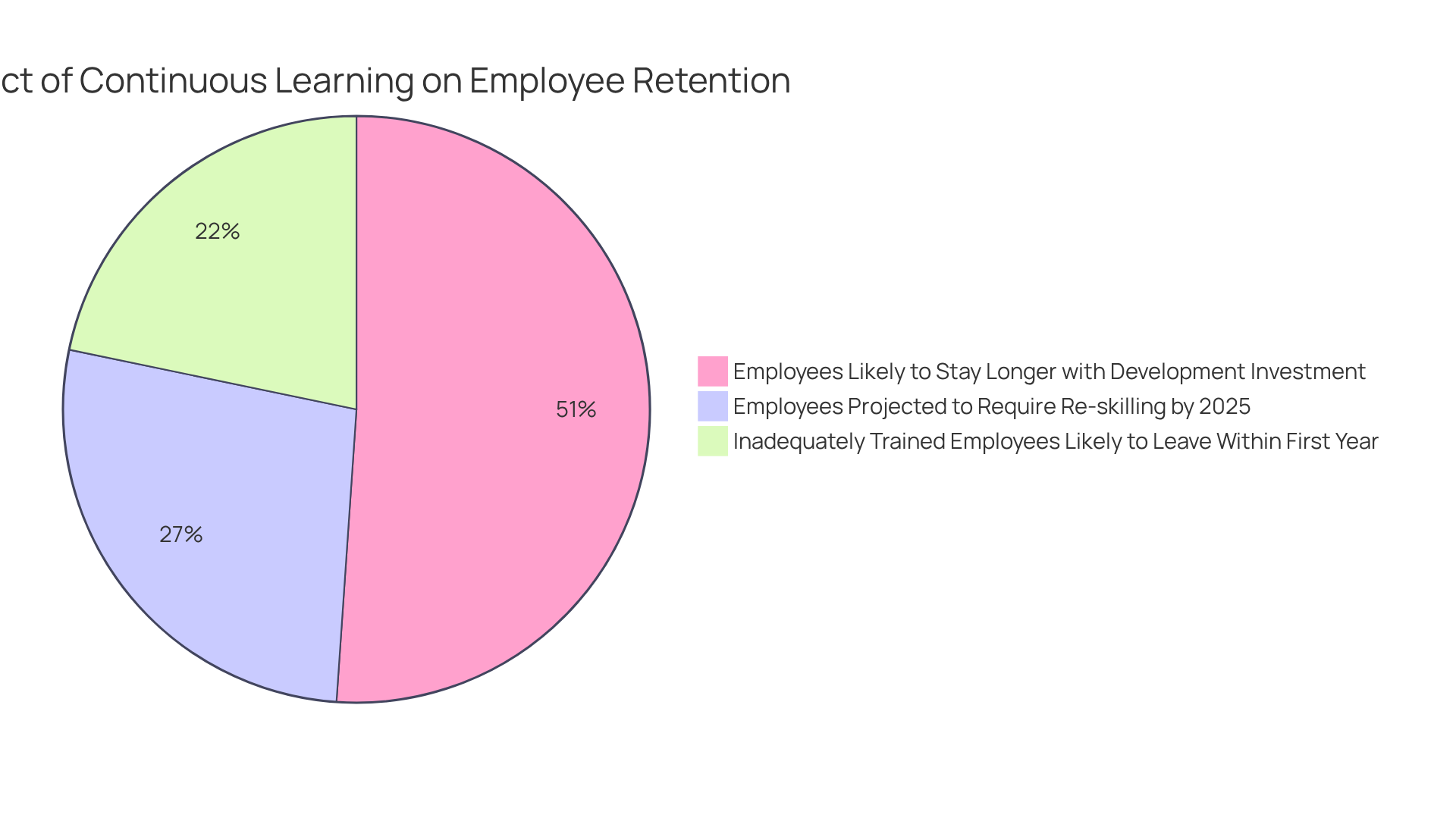
- 94% of employees are likely to stay longer at a company that invests in their learning and development, highlighting the critical role of continuous education in talent retention.
- Projections suggest that 50% of employees will require re-skilling by 2025, the urgency for effective learning strategies has never been more pronounced.
- 40% of employees who receive inadequate training tend to leave their company within the first year, underscoring the repercussions of insufficient training.
As the biologics market is anticipated to grow at a compound annual growth rate of 15% until 2027, it is imperative for professionals to prioritize their growth to adeptly navigate evolving standards and maintain compliance. Albert Einstein aptly stated, "Once you stop learning, you start dying," which underscores the necessity of continuous education in this dynamic field.
Conclusion
The landscape of drug safety jobs in 2025 presents a complex interplay of regulatory compliance, risk management, data analysis, and ethical considerations. As the industry evolves, the essential skills required to excel in this field are increasingly specialized. Professionals must not only grasp the intricacies of pharmacovigilance but also master the tools and methodologies that underpin effective drug safety practices.
Key insights discussed throughout this article underscore the critical importance of communication, attention to detail, and project management in ensuring the safety and efficacy of pharmaceutical products. The role of AVS Life Sciences stands out as a pivotal player, providing the expertise and support necessary to navigate the regulatory landscape and enhance drug safety initiatives. Continuous learning and adaptation to emerging technologies are vital components for success, ensuring professionals remain equipped to meet the demands of an ever-changing environment.
In summary, the future of drug safety jobs is contingent upon a steadfast commitment to excellence, collaboration, and ethical integrity. As the industry confronts new challenges and opportunities, professionals are urged to embrace ongoing education and proactive engagement with the latest advancements. By fostering a culture of continuous improvement and adhering to high standards, the pharmaceutical sector can enhance patient safety and uphold public trust, ultimately contributing to a healthier society.
Frequently Asked Questions
What services does AVS Life Sciences provide?
AVS Life Sciences offers drug safety jobs and medication monitoring services, including validation, regulatory compliance, and quality management for the pharmaceutical and biotechnology sectors.
How many professionals work at AVS Life Sciences?
AVS Life Sciences has a dedicated team of over 300 seasoned professionals.
What is the projected growth of the global drug safety monitoring market?
The global drug safety monitoring market is projected to reach USD 14.03 billion by 2032, with a compound annual growth rate (CAGR) of 8.3% from 2025 to 2032.
Why is drug safety important in public health?
Drug safety is crucial for safeguarding public health and safety, especially as oversight intensifies and the significance of monitoring drug safety escalates.
What qualifications do personnel need to meet regulatory requirements?
National competent authorities and the European Medicines Agency (EMA) mandate that personnel involved in drug safety must be qualified.
What role does the Eudravigilance database play in drug safety?
The Eudravigilance database disseminates drug safety information among competent authorities, emphasizing the importance of regulatory compliance.
What are the key compliance standards relevant to drug safety jobs?
Key compliance standards include Good Manufacturing Practices (GMP), International Organization for Standardization (ISO) standards, and Quality System Regulations (QSR).
What challenges does the pharmaceutical industry face regarding compliance?
The pharmaceutical industry faces significant compliance challenges, as highlighted by the FDA issuing thousands of observations for noncompliance.
How does AVS Life Sciences assist organizations with compliance?
AVS Life Sciences provides extensive consulting services to help organizations navigate the complexities of compliance and quality management in drug safety.
What is the significance of risk management in pharmaceuticals?
Risk management involves identifying, evaluating, and mitigating potential health risks associated with medicinal products, which is vital for patient safety throughout the drug lifecycle.
How has advanced analytics impacted risk management in pharmaceuticals?
The use of advanced analytics in risk management has led to significant reductions in negative event reporting durations and costs associated with preventable adverse events.
Can you provide an example of effective risk management?
A study across 42 hospitals reported a 42% reduction in medication errors, preventing approximately 1.2 million errors annually, demonstrating the effectiveness of structured risk management plans.
How does AVS Life Sciences support risk management strategies?
AVS Life Sciences provides comprehensive quality management and regulatory compliance solutions that help bolster risk management initiatives and improve patient well-being.
