10 Essential Skills Every CQV Engineer Must Master

Overview
The essential skills that every CQV engineer must master encompass:
- Regulatory compliance expertise
- Project management skills
- Technical writing proficiency
- Problem-solving abilities
- Teamwork
- Adaptability
These competencies are not merely beneficial; they are crucial for ensuring successful project execution and compliance within the pharmaceutical industry. By mastering these skills, engineers can adeptly navigate complex regulatory landscapes, manage projects effectively, and produce clear documentation that adheres to industry standards. This mastery ultimately leads to enhanced project outcomes and fosters a culture of compliance that is vital in this highly regulated field.
Introduction
In the fast-paced world of pharmaceuticals, the role of a CQV engineer is pivotal, where regulatory compliance and quality assurance are paramount. Mastering a diverse set of skills not only enhances individual performance but also safeguards the integrity of entire projects. As the landscape of regulations and technologies evolves, CQV engineers must ensure they remain at the forefront of their field to effectively tackle the challenges that arise. This article delves into ten essential skills that every CQV engineer must master to navigate these complexities and drive successful outcomes in their initiatives.
Regulatory Compliance Expertise: Essential for CQV Engineers
CQV professionals must possess a profound understanding of regulatory frameworks, particularly Good Manufacturing Practices (GMP), ISO standards, and Quality System Regulations (QSR). This expertise is crucial for ensuring that all processes and systems comply with necessary requirements, significantly reducing the risk of regulatory penalties, which can average $5.05 million for organizations with high non-compliance.
Furthermore, familiarity with these regulations facilitates the preparation of essential documentation and audits—integral components of the CQV process. As the regulatory landscape evolves, staying informed on GMP standards in 2025 is essential, especially as 70% of organizations indicate the necessity to demonstrate adherence to various frameworks.
Industry leaders emphasize that effective adherence not only safeguards product safety but also enhances operational efficiency, positioning it as a strategic priority for the CQV engineer. The financial implications of non-compliance underscore the importance of rigorous adherence to these standards, as penalties can severely impact pharmaceutical companies' bottom lines.
To remain informed, CQV professionals should regularly engage with industry publications, attend relevant training sessions, and participate in professional networks focused on .
Project Management Skills: Key to Successful CQV Execution
CQV engineers are essential in overseeing the stages of commissioning, qualification, and validation, which requires strong management skills. Their expertise in creating detailed plans, managing timelines, and collaborating with cross-functional teams is essential. Efficient management of tasks guarantees that all activities conducted by the CQV engineer are completed on time and within budget, while also upholding regulations and meeting objectives.
A notable example of this is AVS Life Sciences' recent initiative, which successfully upgraded a biotechnology GMP facility from Biosafety Level 1 to Level 2. This endeavor was completed on time and within financial limits, showcasing the significance of careful management in attaining . Throughout this initiative, specific methodologies, such as agile practices, were utilized to enhance flexibility and adaptability in response to changing requirements.
Furthermore, the team gained important insights regarding irregularities in test outcomes, emphasizing the necessity for enhanced testing procedures and comprehensive inspections of equipment configuration. Recent advancements in management tools, including intuitive software that enhances team collaboration and communication, are transforming the execution of processes for the CQV engineer.
Organizations that employ organized management practices report a 2.5 times greater success rate in achieving their original objectives, underscoring the essential nature of efficient management in the role of a CQV engineer. Optimal management approaches for the CQV engineer execution involve adopting agile methodologies, which permit flexibility and adaptability in response to evolving requirements. Additionally, it is crucial to acknowledge that merely 27% of organizations consistently utilize risk management practices, highlighting the importance of incorporating risk management into CQV management.
As the life sciences sector increasingly adopts digital tools, CQV professionals must remain informed about current trends to enhance project outcomes and promote efficiency.
Technical Writing Proficiency: Critical for Documentation in CQV
Proficiency in technical writing is essential for a CQV engineer, who is tasked with creating and maintaining vital documentation, including:
Clear and precise documentation not only ensures compliance with regulatory requirements but also serves as a crucial reference during audits and inspections. Experts in the field emphasize that well-organized documentation can significantly reduce the time spent on revisions and enhance overall efficiency.
Engineers must prioritize the development of standardized templates and guidelines to streamline the documentation process, ensuring consistency and clarity across all materials. Effective validation protocols and SOPs are indispensable, as they provide a framework for adherence and operational excellence in the life sciences sector.
On average, CQV engineer initiatives allocate a substantial portion of their timeline to documentation, highlighting its critical role in the engineering process. By mastering technical writing, CQV engineers can foster a culture of compliance and quality assurance that is vital for achieving successful project outcomes.
Problem-Solving Abilities: Vital for Overcoming Validation Challenges
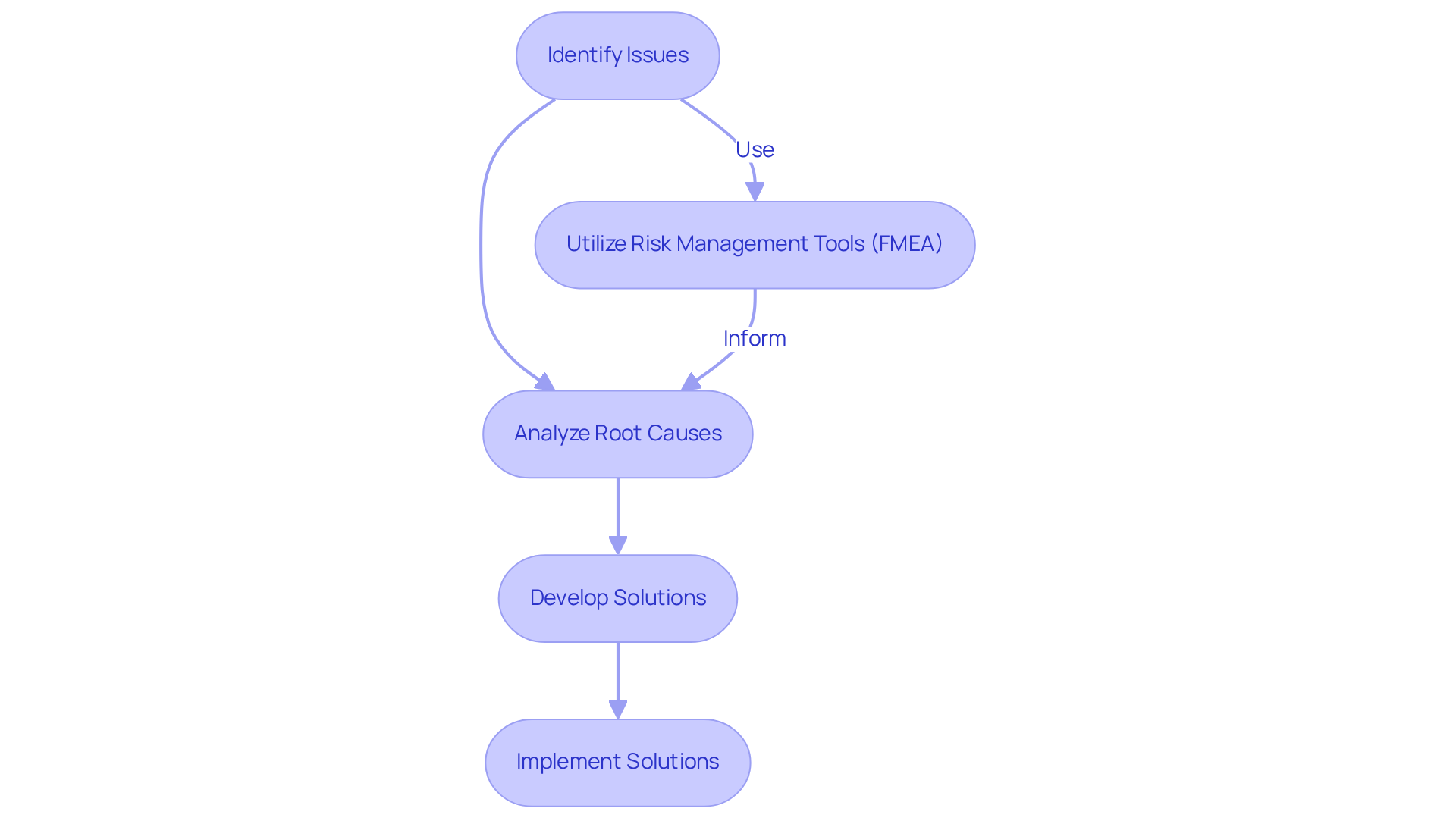
Problem-solving abilities are essential for a cqv engineer, as they frequently encounter complex challenges during the validation process. These professionals must excel in identifying issues, analyzing root causes, and implementing effective solutions. For instance, utilizing risk management tools like Failure Mode and Effects Analysis (FMEA) allows engineers to anticipate potential problems and develop contingency plans. This proactive approach not only enhances the efficiency of initiatives but also ensures compliance with regulatory standards.
A notable case of effective problem-solving can be observed in the work of AVS Life Sciences, which supported a leading biotechnology company in upgrading its manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. Throughout this project, demonstrated exceptional problem-solving skills, addressing challenges such as ensuring complete traceability and adherence to quality assurance standards. Their meticulous documentation efforts and support during the transition enabled the client to concentrate on developing medicines for serious diseases, ultimately improving the quality of life for patients.
Industry leaders underscore the importance of confronting validation challenges directly. As Stacy Capek, Director of Quality & Training at Verista, aptly stated, 'There’s never time to do it right the first time, but always time to do it over.' This highlights the necessity of comprehensive problem-solving strategies that can prevent costly rework and ensure timely delivery. By mastering these skills, a cqv engineer can significantly enhance the quality and efficiency of validation processes, ultimately contributing to the success of pharmaceutical endeavors.
Teamwork and Collaboration: Enhancing CQV Project Success
Teamwork and collaboration are paramount for the success of CQV initiatives. CQV engineers must engage closely with various stakeholders, including:
- Quality assurance
- Engineering
- Regulatory teams
to ensure alignment with regulatory requirements. Regular meetings and open communication channels facilitate information sharing and problem-solving, which are essential for effective execution. This not only streamlines workflows but also significantly enhances compliance outcomes in pharmaceutical initiatives.
Recent trends indicate a growing reliance on advanced collaboration tools tailored for life sciences teams, enabling real-time communication and task management. By leveraging these tools, teams can improve their coordination, ultimately leading to higher success rates and adherence to regulatory standards.
Insights from industry leaders highlight that organizations prioritizing collaboration are more likely to meet their project goals, reinforcing the critical role of teamwork in CQV engineer projects.
Good Manufacturing Practices (GMP) Knowledge: Foundation for Quality Assurance
A solid understanding of Good Manufacturing Practices (GMP) is crucial for a CQV engineer, as it forms the foundation of quality assurance in the pharmaceutical industry. A CQV engineer must be familiar with GMP guidelines to ensure that all processes, equipment, and facilities meet the required standards for safety and efficacy. This knowledge empowers engineers to identify potential regulatory gaps and implement corrective measures to uphold product quality during the manufacturing process.
For instance, AVS Life Sciences recently assisted a pharmaceutical manufacturer in upgrading their facility from a Biosafety Level 1 to a Level 2 GMP-compliant environment. This project involved:
- A comprehensive gap analysis
- Equipment procurement
- The installation of over 80 new pieces of equipment, all while adhering to stringent GMP standards.
Such experiences underscore the critical role that GMP knowledge plays in ensuring successful compliance and quality assurance in .
Validation Methodologies: Core Competency for CQV Engineers
CQV specialists must possess a profound understanding of various validation methodologies, particularly Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). Mastery of these methodologies empowers professionals to design and implement robust validation strategies that confirm systems and processes function as intended. The success rates of diverse validation approaches in CQV projects significantly influence overall project outcomes; effective execution leads to improved adherence and a reduced risk of non-conformance.
Incorporating risk-based validation approaches enhances the efficiency of the validation process. By prioritizing high-risk areas, professionals can allocate resources more effectively, ensuring that critical aspects of the system receive the necessary attention while still adhering to regulatory requirements. This strategic focus streamlines the and contributes to maintaining high standards of quality and compliance throughout the product lifecycle.
Recent updates in the field underscore the importance of thorough documentation and adherence to established guidelines during IQ, OQ, and PQ phases. These updates reflect the evolving regulatory landscape and the necessity for continuous improvement in validation practices. As the pharmaceutical sector faces increasing demands for quality assurance, the role of the CQV engineer in executing effective validation strategies becomes increasingly vital.
Adaptability: Essential for Navigating Industry Changes
Adaptability is paramount for a CQV engineer, who must adeptly navigate a dynamic regulatory landscape and technological advancements. Staying informed about industry trends and emerging technologies is essential for maintaining competitiveness and compliance. Ongoing education and training are crucial, enabling professionals to cultivate a mindset of adaptability and enhancement. This proactive approach allows them to respond effectively to , significantly improving success rates.
For instance, recent changes in regulatory requirements have necessitated that professionals develop robust strategies for compliance, ensuring their initiatives align with evolving standards. Industry leaders emphasize that a well-prepared CQV engineer, who possesses the latest knowledge and skills, is vital for successfully managing compliance-driven projects.
By fostering a culture of adaptability, CQV professionals can not only meet current demands but also anticipate future changes, positioning themselves as invaluable assets in the life sciences sector.
Analytical Skills: Important for Data-Driven Decision Making
Analytical abilities are essential for a CQV engineer, enabling them to effectively scrutinize data from diverse sources to inform validation and adherence decisions. Proficiency in statistical analysis and data interpretation is vital for identifying trends, assessing risks, and evaluating the effectiveness of validation efforts.
For example, engineers typically allocate 60% to 80% of their time to data retrieval, highlighting the necessity for efficient data management practices, as illustrated by AVS Life Sciences in their transformative case study of upgrading a biotechnology GMP facility. This project was completed on schedule and within budget, showcasing AVS's expertise in ensuring compliance and operational excellence while underscoring the in the manufacturing process.
Recent advancements in data analytics, particularly following AVS's acquisition of ValidPath, have streamlined validation processes, facilitating quicker insights and enhanced accuracy in decision-making. Moreover, the integration of data-driven methodologies not only improves project outcomes but also aligns with the industry's transition towards evidence-based practices.
Industry professionals assert that effective decision-making hinges on clean, well-analyzed data, reinforcing the idea that reliable data is crucial for successful outcomes. Consequently, a CQV engineer must cultivate robust analytical skills to navigate the complexities of validation and ensure adherence to regulatory standards.
Continuous Learning: Key to Staying Current in CQV Engineering
Continuous learning is essential for CQV engineers to stay proficient in the rapidly changing landscape of regulations, technologies, and industry standards. At AVS Life Sciences, we are dedicated to your growth, providing substantial personal and professional development through a variety of opportunities, including:
- Workshops
- Webinars
- Industry conferences
For example, our webinars offer in-depth analyses of the FDA's Data Integrity Guidance, enabling teams to grasp the critical role that data integrity plays in business decision-making and compliance. Engaging in ongoing education not only sharpens personal skills but also significantly enhances the effectiveness of initiatives led by a CQV engineer.
Research indicates that companies prioritizing continuous education experience a 17% increase in productivity and a 21% rise in profits, underscoring the direct correlation between employee development and organizational outcomes. Moreover, businesses that allocate resources to learning witness a remarkable 56% increase in revenue compared to those that do not.
As Isidora Markovic aptly notes, "The investment you make in your people through providing timely, regular learning opportunities will be returned tenfold in their productivity and output." This unwavering commitment to equips CQV engineers with the latest knowledge and tools required to adeptly navigate compliance challenges, ultimately driving project success.
Conclusion
Mastering the essential skills for CQV engineers is crucial for navigating the complexities of the life sciences industry. Proficiency in regulatory compliance, project management, technical writing, problem-solving, teamwork, and adaptability not only enhances the effectiveness of CQV initiatives but also contributes significantly to achieving compliance and maintaining product quality. As the landscape of regulations and technologies evolves, these competencies become increasingly vital for ensuring successful project outcomes and safeguarding public health.
The importance of each skill is underscored throughout the article.
- Regulatory compliance expertise ensures adherence to necessary standards.
- Project management skills facilitate effective execution of CQV processes.
- Technical writing proficiency is essential for clear documentation.
- Strong problem-solving abilities allow engineers to tackle challenges head-on.
- Teamwork and collaboration enhance communication among stakeholders.
- Adaptability ensures that CQV professionals can respond swiftly to industry changes.
- Analytical skills empower engineers to make informed, data-driven decisions, reinforcing the need for continuous learning in this dynamic field.
In summary, the role of a CQV engineer is multifaceted, requiring a diverse skill set to thrive in an ever-evolving industry. Embracing ongoing education and professional development not only fosters personal growth but also positions CQV engineers as invaluable assets within their organizations. As the life sciences sector continues to advance, the commitment to mastering these essential skills will enhance individual careers and contribute to the overall success and integrity of pharmaceutical initiatives.
Frequently Asked Questions
What regulatory frameworks must CQV professionals understand?
CQV professionals must possess a profound understanding of Good Manufacturing Practices (GMP), ISO standards, and Quality System Regulations (QSR) to ensure compliance with necessary requirements.
Why is regulatory compliance important for CQV engineers?
Regulatory compliance is crucial for reducing the risk of penalties, which can average $5.05 million for organizations with high non-compliance. It also safeguards product safety and enhances operational efficiency.
How can CQV professionals stay informed about regulatory changes?
CQV professionals can stay informed by engaging with industry publications, attending relevant training sessions, and participating in professional networks focused on compliance updates.
What project management skills are essential for CQV engineers?
CQV engineers need strong management skills for creating detailed plans, managing timelines, and collaborating with cross-functional teams to ensure tasks are completed on time and within budget.
Can you provide an example of successful project management in CQV?
An example is AVS Life Sciences' initiative to upgrade a biotechnology GMP facility from Biosafety Level 1 to Level 2, which was completed on time and within budget, showcasing the importance of careful management.
What methodologies can enhance management flexibility in CQV?
Agile methodologies can enhance flexibility and adaptability in response to changing requirements during the CQV process.
What role does technical writing play in the work of a CQV engineer?
Technical writing is essential for creating and maintaining documentation such as validation protocols, reports, and standard operating procedures (SOPs), ensuring compliance and serving as a reference during audits.
How can effective documentation impact CQV initiatives?
Well-organized documentation can significantly reduce revision time and enhance overall efficiency, fostering a culture of compliance and quality assurance.
What percentage of their timeline do CQV engineers typically allocate to documentation?
CQV engineer initiatives typically allocate a substantial portion of their timeline to documentation, highlighting its critical role in the engineering process.
What is the importance of standardized templates and guidelines in technical writing for CQV?
Standardized templates and guidelines help streamline the documentation process, ensuring consistency and clarity across all materials, which is vital for compliance and operational excellence.
