10 Essential Puerto Rico Validation Services for Compliance Officers

Overview
The article outlines critical Puerto Rico validation services that compliance officers in the pharmaceutical sector must leverage to ensure adherence to regulatory standards. It underscores the significance of services such as:
These services not only help maintain compliance with stringent regulations but also enhance operational efficiency throughout the drug development lifecycle. By employing these essential validation services, organizations can effectively navigate the complexities of compliance, thereby fostering a culture of excellence and reliability in their operations.
Introduction
The pharmaceutical landscape is increasingly complex, presenting compliance officers with mounting pressures to ensure adherence to stringent regulatory standards. In this challenging environment, understanding the essential validation services available in Puerto Rico is paramount for maintaining both quality and compliance. Critical services exist that can empower these professionals to navigate the intricate web of regulations. By leveraging these resources, they can enhance operational efficiency and uphold product integrity.
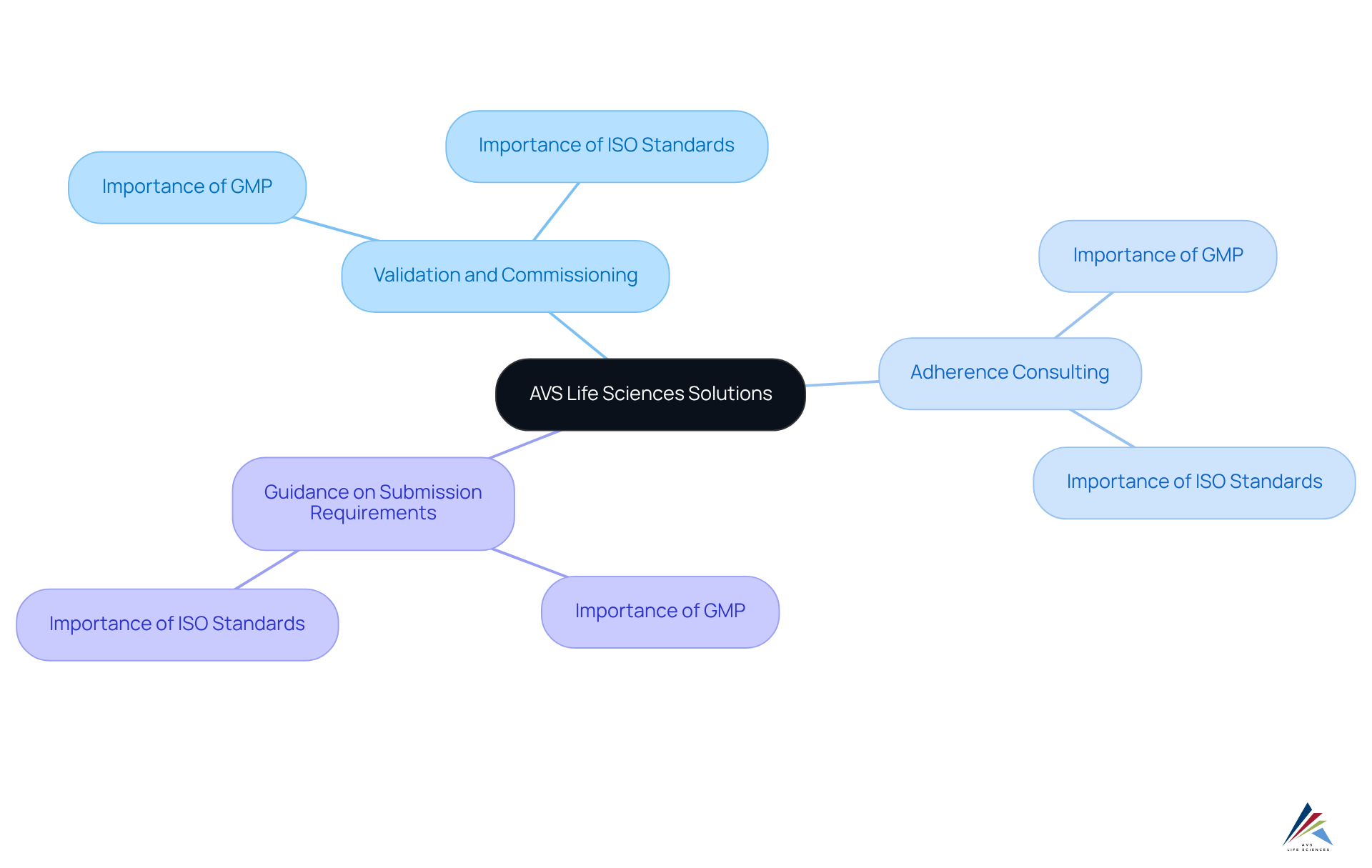
AVS Life Sciences: Comprehensive Validation and Quality Compliance Solutions
AVS Life Sciences delivers a comprehensive array of validation and standards adherence solutions meticulously designed for the pharmaceutical, biotechnology, and medical device sectors. Their offerings encompass:
By placing a strong emphasis on Good Manufacturing Practices (GMP) and ISO standards, AVS Life Sciences is committed to empowering clients throughout the entire product lifecycle. Their expertise in ensures compliance in APIs, drug products, and testing facilities, positioning them as a trusted partner for regulatory officers aiming to enhance GMP facilities and uphold standards.
Advanced Telematics Solutions for Regulatory Compliance
AVS Life Sciences presents a comprehensive suite of advisory services designed to ensure compliance with industry standards and facilitate effective management within the biopharmaceutical and life sciences sectors. Our extensive expertise spans GXP standards, FDA regulations, and the formulation of Standard Operating Procedures (SOPs). We prioritize , conducting meticulous investigations, and implementing Corrective and Preventive Actions (CAPA) to satisfy the rigorous demands of the industry.
Leveraging our profound knowledge of both internal and external auditing methods, we empower organizations to navigate the complexities of regulatory frameworks while simultaneously enhancing their operational efficiency. By showcasing successful compliance projects, we illustrate our capability to deliver tailored solutions that resonate with your specific needs, prompting you to engage with AVS Life Sciences for unparalleled support in achieving compliance excellence.

Precision Calibration Services for Pharmaceutical Applications
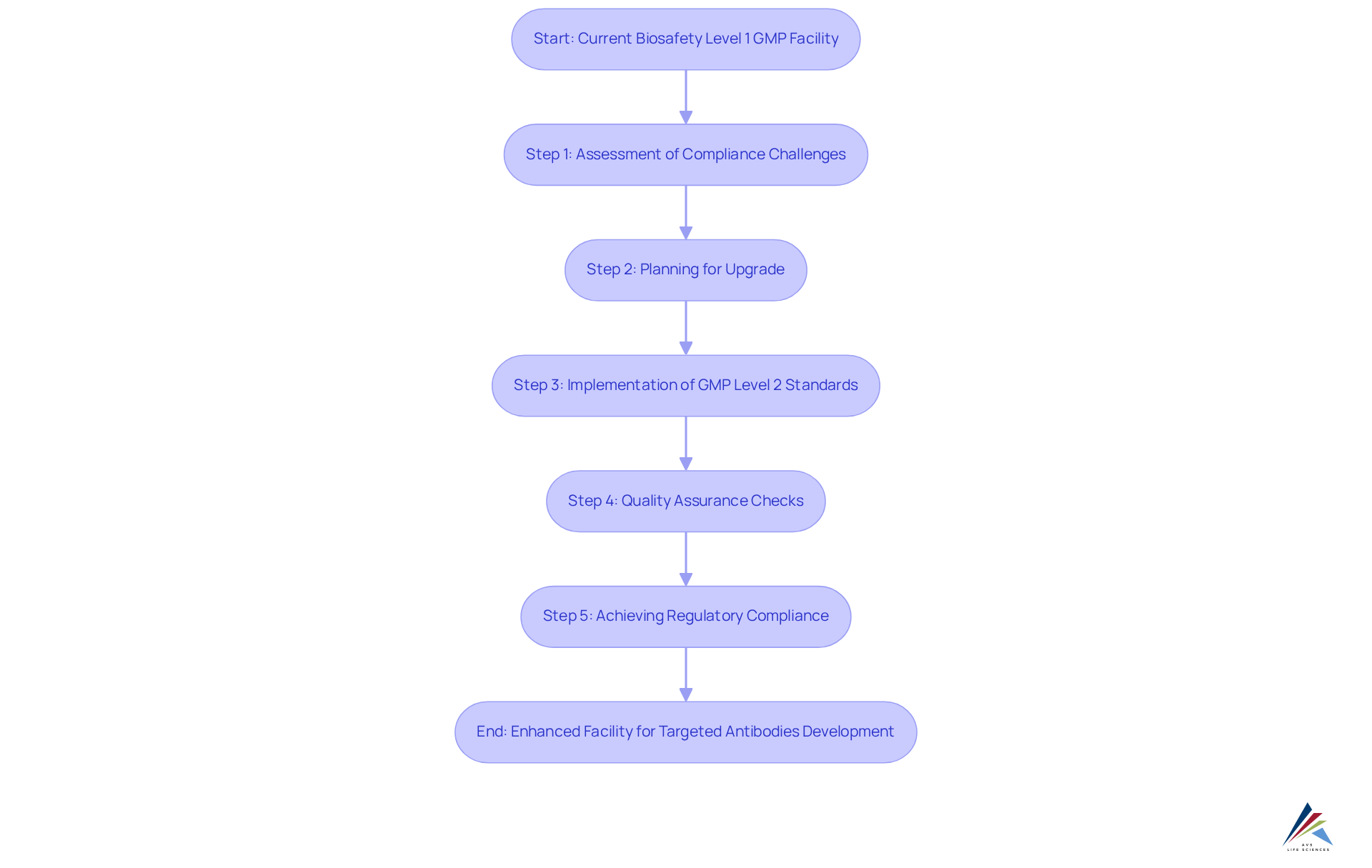
AVS Life Sciences addresses the critical compliance challenges faced by the pharmaceutical sector through comprehensive management of standards and regulatory adherence solutions. Their recent case study exemplifies a successful upgrade of a biotechnology GMP facility, underscoring the vital role of quality assurance throughout the process.
By assisting a leading biotechnology firm in San Francisco in transitioning from a Biosafety Level 1 GMP facility to a Level 2 GMP facility, AVS Life Sciences demonstrated their commitment to enhancing adherence and achieving operational excellence. This upgrade not only but also empowered the client to focus on developing targeted antibodies for serious diseases, ultimately improving patient outcomes.
As we look to 2025, the significance of such transformative enhancements is evident; adherence rates indicate that organizations prioritizing management solutions achieve a significantly higher success rate in meeting compliance standards.
AVS Life Sciences' unwavering dedication to quality assurance and adherence to regulations positions them as a trusted partner in the life sciences sector, reinforcing the essential nature of these services in ensuring operational excellence.
Expert Validation Services for Compliance
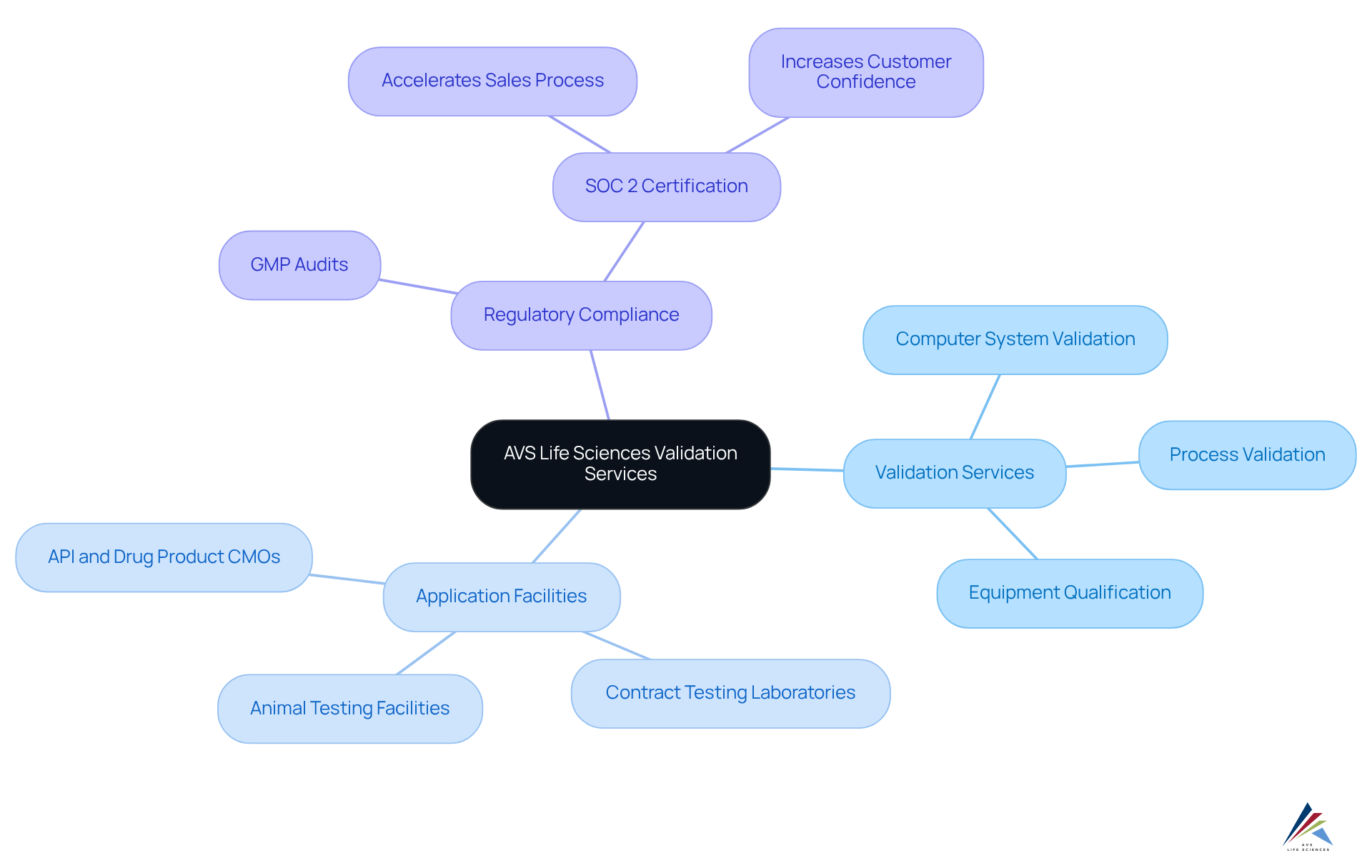
AVS Life Sciences provides specialized Puerto Rico validation services that are crucial for ensuring compliance within the pharmaceutical sector. Their extensive offerings of Puerto Rico validation services include:
All meticulously designed to ensure that systems and processes meet rigorous regulatory standards. Notably, AVS Life Sciences conducts GMP audits across various sites, including:
Thereby ensuring compliance in critical phases of the drug development lifecycle. By leveraging their vast expertise, compliance officers can effectively manage Puerto Rico validation services, ensuring that all facets of regulatory requirements are thoroughly addressed. This proactive approach not only streamlines the validation process but also aligns with contemporary trends that underscore the in maintaining quality and compliance.
Furthermore, as emphasized by Sriram Gopalan, the confidence derived from certifications like SOC 2 can significantly expedite the sales process, highlighting the intrinsic value of proficient validation services. Regulatory officers are urged to remain abreast of these trends and consider how they can utilize AVS Life Sciences' services to enhance their regulatory strategies.
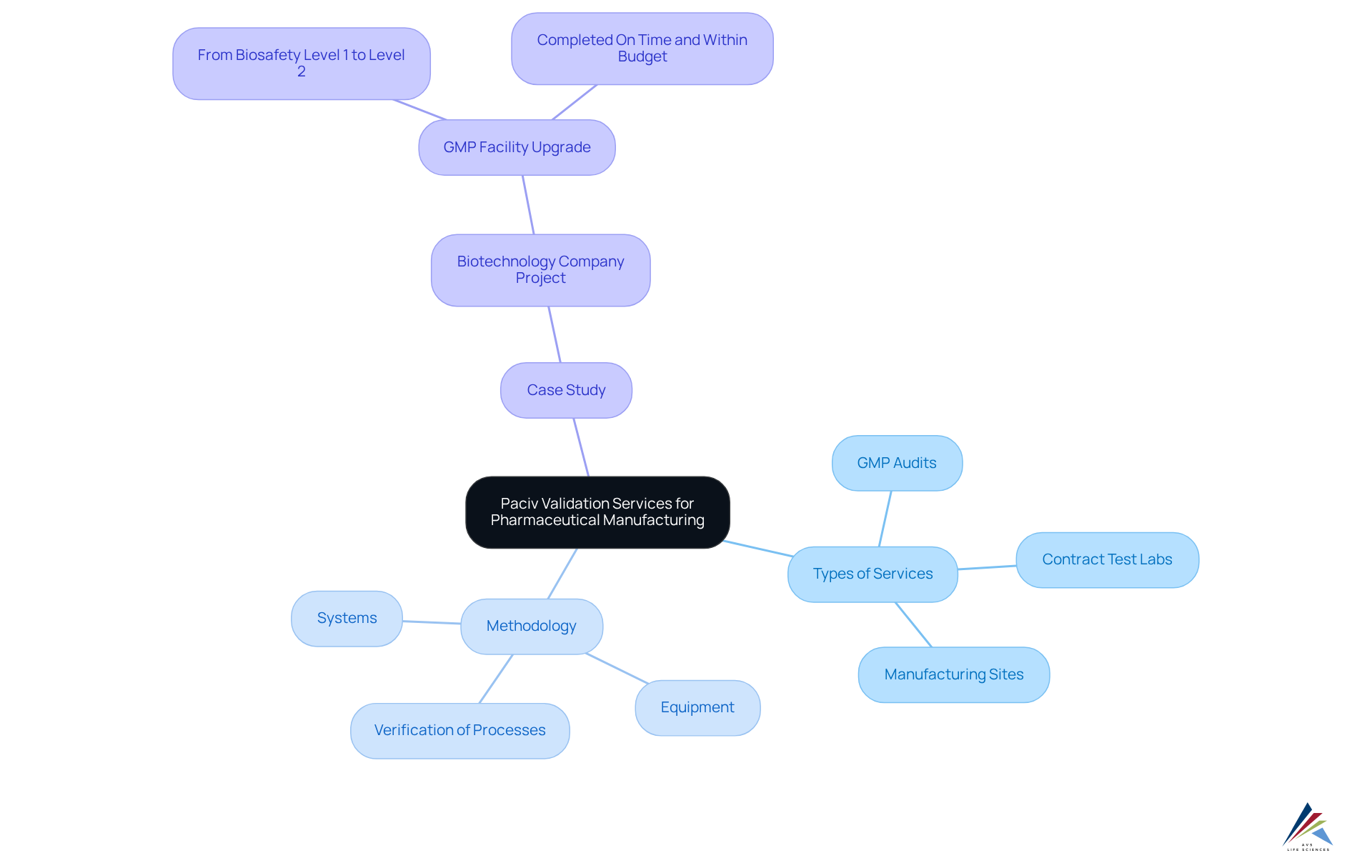
Tailored Validation Services for Pharmaceutical Manufacturing
AVS Life Sciences offers tailored Puerto Rico validation services specifically designed for pharmaceutical manufacturing, encompassing GMP audits for API and drug product CMOs, contract test labs, and manufacturing sites. Their comprehensive methodology includes the verification of processes, equipment, and systems, ensuring strict adherence to legal standards.
By delivering customized solutions, AVS Life Sciences aids manufacturers in navigating the complexities of regulatory compliance while simultaneously enhancing operational efficiency. A compelling illustration of their expertise is demonstrated in a recent case study where AVS supported a leading biotechnology company in upgrading their GMP facility from Biosafety Level 1 to Level 2. This project was completed on time and within budget, underscoring AVS's unwavering commitment to and compliance with standards throughout the drug development lifecycle.
Validation Specialist: Key Role in Ensuring Pharmaceutical Compliance
Validation experts are pivotal in within the pharmaceutical industry. They are responsible for developing and executing validation protocols, conducting audits, and ensuring that all processes meet compliance standards. Their expertise is crucial for maintaining product quality and safety, making them indispensable to regulatory teams.
At AVS Life Sciences, professionals like Nykkytta Mendez exemplify a commitment to meticulous management and adherence to regulations. Their efforts ensure that validation processes not only align with industry standards but also embody best practices. This dedication to compliance not only safeguards public health but also enhances the reputation of the pharmaceutical sector.
Engaging with AVS Life Sciences means partnering with a leader in compliance solutions, including Puerto Rico validation services, where expertise meets an unwavering commitment to excellence.
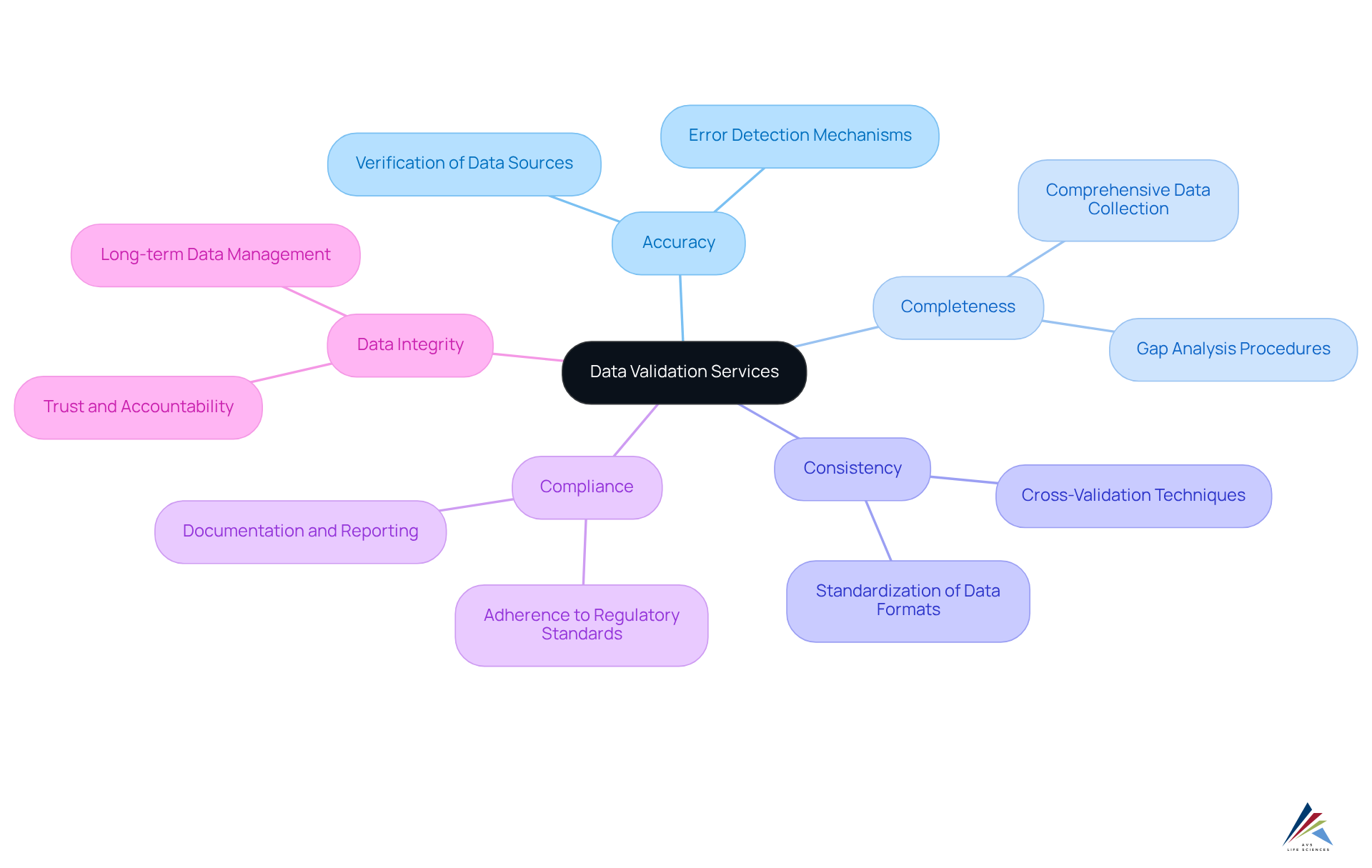
Data Validation Services: Ensuring Integrity in Pharmaceutical Data
Data validation services play a crucial role in safeguarding the integrity of pharmaceutical data. These services entail a meticulous verification process, ensuring the accuracy, completeness, and consistency of data gathered during research and manufacturing. By implementing rigorous data validation procedures, companies not only bolster their compliance with regulatory standards but also significantly enhance overall data integrity. This commitment to is essential for maintaining trust and accountability in the pharmaceutical sector.
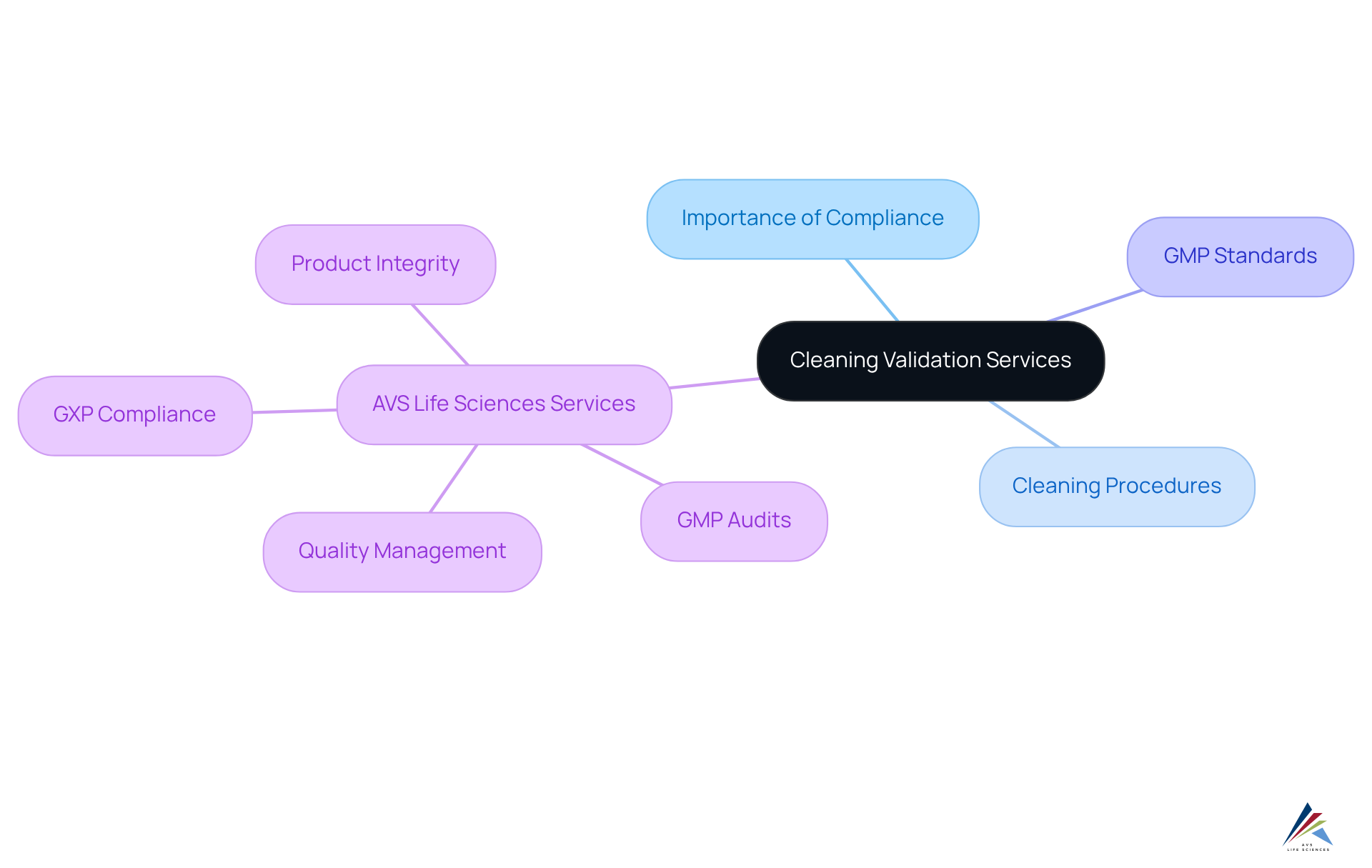
Cleaning Validation Services: Maintaining Compliance in Pharmaceutical Environments
Puerto Rico validation services are crucial for ensuring compliance within pharmaceutical environments. These services guarantee that cleaning procedures effectively eliminate residues and contaminants from equipment and surfaces, thereby preventing cross-contamination. By adhering to rigorous cleaning validation protocols, pharmaceutical firms can ensure compliance with GMP standards through Puerto Rico validation services and safeguard product integrity.
AVS Life Sciences offers comprehensive GXP compliance services, including GMP audits focused on APIs, drug products, and testing facilities. Their extensive expertise in quality management and strict adherence to regulations ensures that Puerto Rico validation services align with industry standards. This alignment not only but also reinforces safety in pharmaceutical operations.
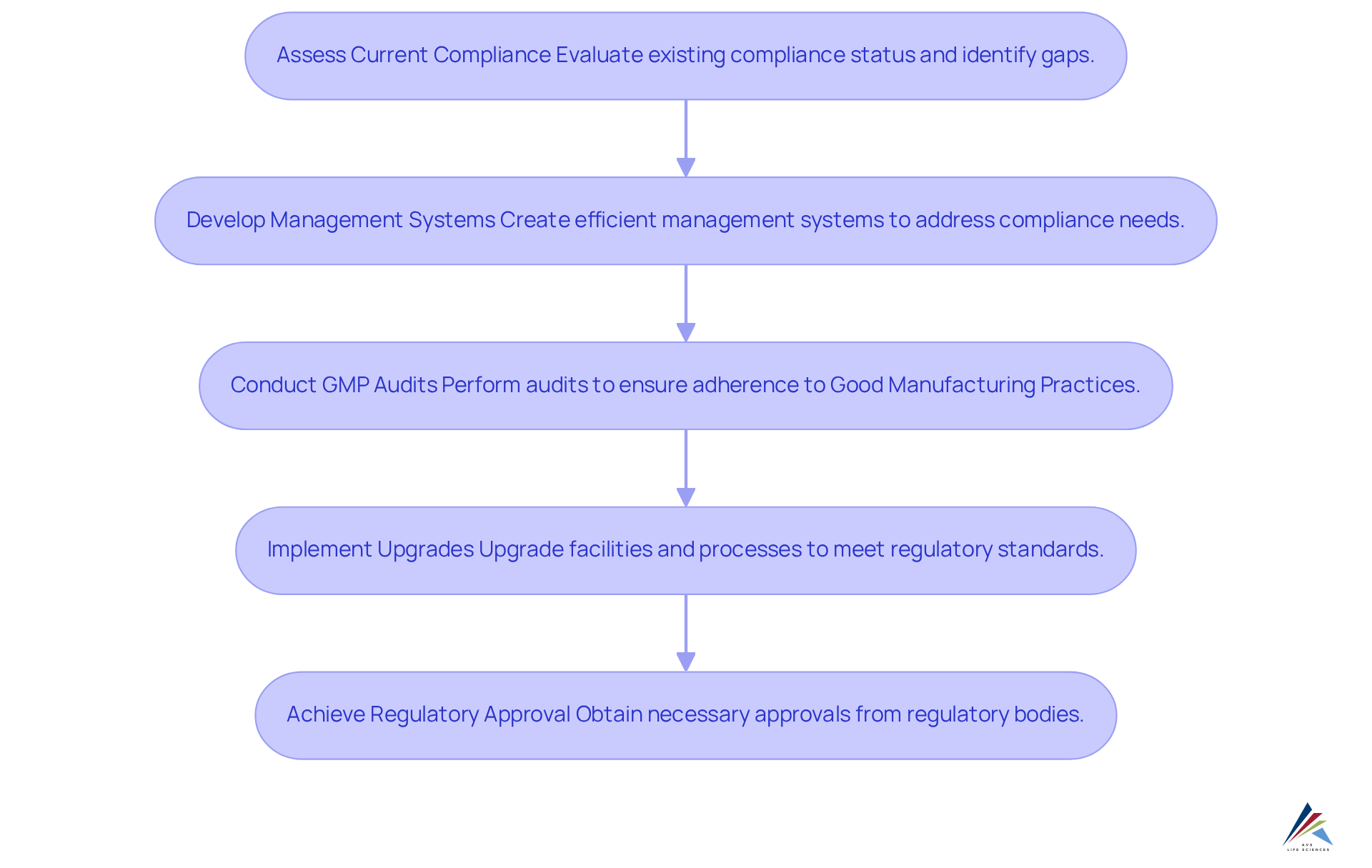
Quality Compliance Consulting: Navigating Regulatory Landscapes in Pharmaceuticals
Quality compliance consulting services are essential for pharmaceutical companies navigating the intricate legal environments they face. AVS Life Sciences offers comprehensive GXP compliance services, including GMP audits for APIs, drug products, and testing facilities. Their strategic direction empowers organizations to develop and ensure compliance with legal standards.
A notable case study illustrates AVS's expertise:
By leveraging their extensive knowledge, companies can enhance their compliance initiatives and mitigate risks, ultimately improving the quality of life for patients.
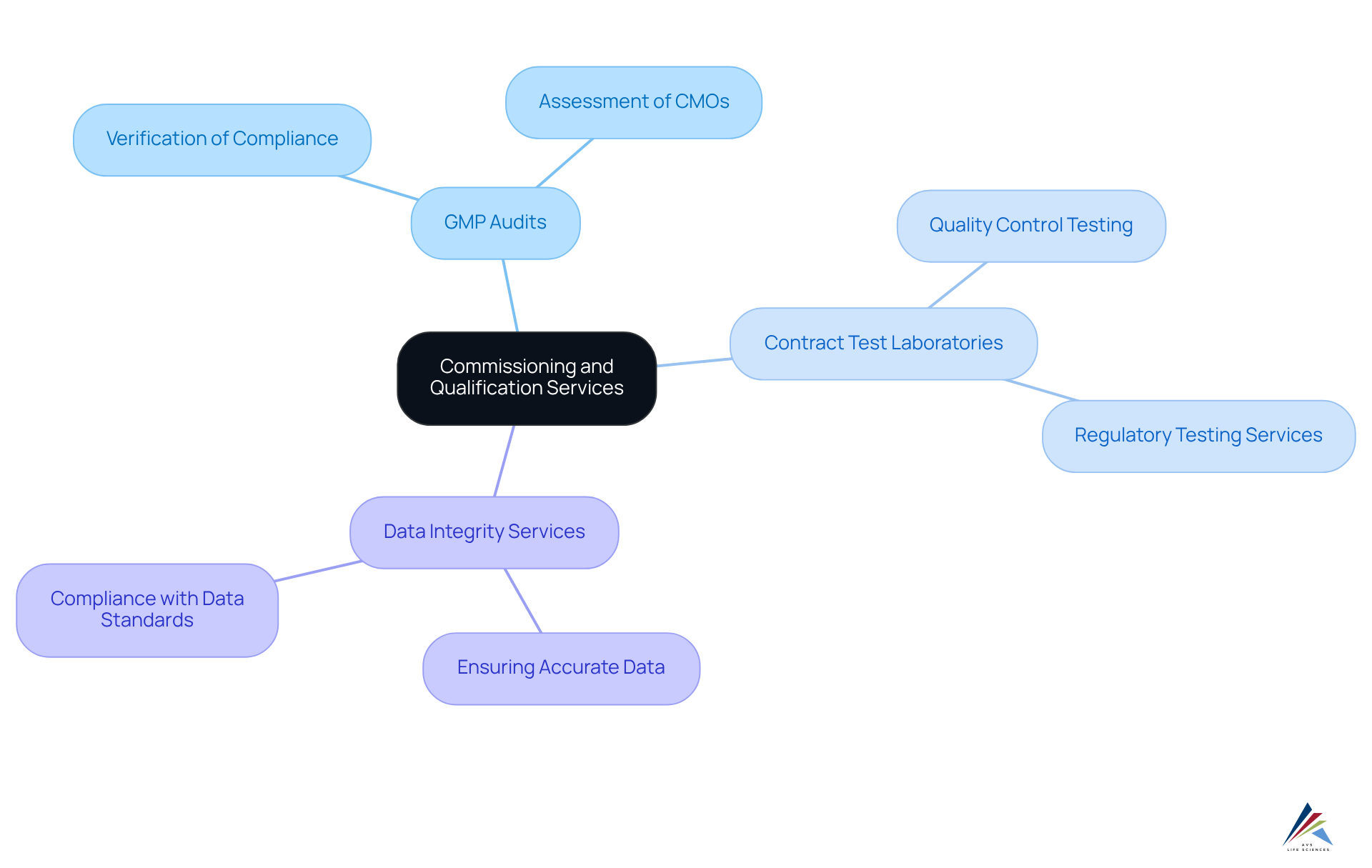
Commissioning and Qualification Services: Ensuring Regulatory Compliance in Pharmaceuticals
Commissioning and qualification services are essential for ensuring compliance with regulations in the pharmaceutical sector. AVS Life Sciences provides comprehensive GXP compliance services, encompassing:
These services are pivotal in verifying that facilities, systems, and equipment are designed, installed, and operate in alignment with regulatory standards. By adopting robust , AVS Life Sciences empowers companies to guarantee that their operations not only meet compliance requirements but also uphold product quality throughout the drug development lifecycle.
Conclusion
AVS Life Sciences emerges as an essential ally for compliance officers in the pharmaceutical sector, offering validation services that are both effective and comprehensive. Their extensive offerings, ranging from GMP audits to customized validation solutions, highlight the critical need to adhere to regulatory standards, thereby ensuring product quality and safety. By harnessing their expertise, organizations can confidently navigate the intricate landscape of compliance, aligning their operations with the highest industry standards.
Key services such as data validation, cleaning validation, and commissioning and qualification are integral to a robust compliance strategy. Each service is pivotal in preserving the integrity of pharmaceutical processes, ensuring that organizations not only meet but surpass regulatory expectations. The success stories and case studies presented further illustrate the tangible benefits of collaborating with AVS Life Sciences, underscoring their unwavering commitment to operational excellence and quality assurance.
In today’s fast-paced regulatory environment, the importance of comprehensive validation services is paramount. Compliance officers are urged to prioritize these essential services to refine their regulatory strategies and ultimately enhance patient outcomes. By partnering with AVS Life Sciences, organizations can position themselves at the forefront of compliance, paving the way for innovation and success within the pharmaceutical industry.
Frequently Asked Questions
What services does AVS Life Sciences provide?
AVS Life Sciences offers a range of validation and standards adherence solutions for the pharmaceutical, biotechnology, and medical device sectors, including validation and commissioning, adherence consulting, and guidance on submission requirements.
How does AVS Life Sciences ensure compliance with industry standards?
AVS Life Sciences emphasizes Good Manufacturing Practices (GMP) and ISO standards, ensuring compliance in APIs, drug products, and testing facilities. They also conduct GMP inspections to support regulatory officers in enhancing GMP facilities.
What expertise does AVS Life Sciences have in regulatory compliance?
AVS Life Sciences has extensive expertise in GXP standards, FDA regulations, and the formulation of Standard Operating Procedures (SOPs), focusing on data integrity and implementing Corrective and Preventive Actions (CAPA) to meet industry demands.
Can you provide an example of AVS Life Sciences' impact in the pharmaceutical sector?
AVS Life Sciences successfully assisted a biotechnology firm in upgrading from a Biosafety Level 1 GMP facility to a Level 2 GMP facility, ensuring compliance with stringent standards and enabling the client to focus on developing targeted antibodies for serious diseases.
What is the significance of AVS Life Sciences' services for organizations in the life sciences sector?
AVS Life Sciences' services are essential for ensuring operational excellence and compliance with regulations, as organizations that prioritize management solutions have a higher success rate in meeting compliance standards.
How does AVS Life Sciences support organizations in navigating regulatory frameworks?
By leveraging their knowledge of internal and external auditing methods, AVS Life Sciences empowers organizations to navigate complex regulatory frameworks while enhancing operational efficiency through tailored compliance solutions.
