10 Essential Insights on SISPQ for Compliance Officers

Overview
The article delivers critical insights on SISPQ (Safety, Identity, Strength, Purity, and Quality) tailored for compliance officers, underscoring its vital role in safeguarding pharmaceutical product integrity and ensuring regulatory adherence. It delineates the key components of SISPQ, accentuates the significance of risk management and documentation practices, and explores emerging trends such as digital transformation and sustainability that compliance officers must adeptly navigate to uphold compliance excellence.
Introduction
In the intricate world of pharmaceuticals, compliance officers confront the formidable challenge of ensuring that products align with the stringent standards encapsulated in the SISPQ framework—Safety, Identity, Strength, Purity, and Quality. This framework transcends mere regulatory requirement; it is a critical element that safeguards patient health and upholds trust in pharmaceutical offerings.
As regulations evolve and new challenges arise, how can compliance officers adeptly navigate this landscape to uphold these vital standards? This article explores ten essential insights on SISPQ, delivering valuable strategies and best practices that empower compliance professionals to excel in their roles and enhance the overall integrity of pharmaceutical products.
AVS Life Sciences: Comprehensive Insights on SISPQ for Compliance Excellence
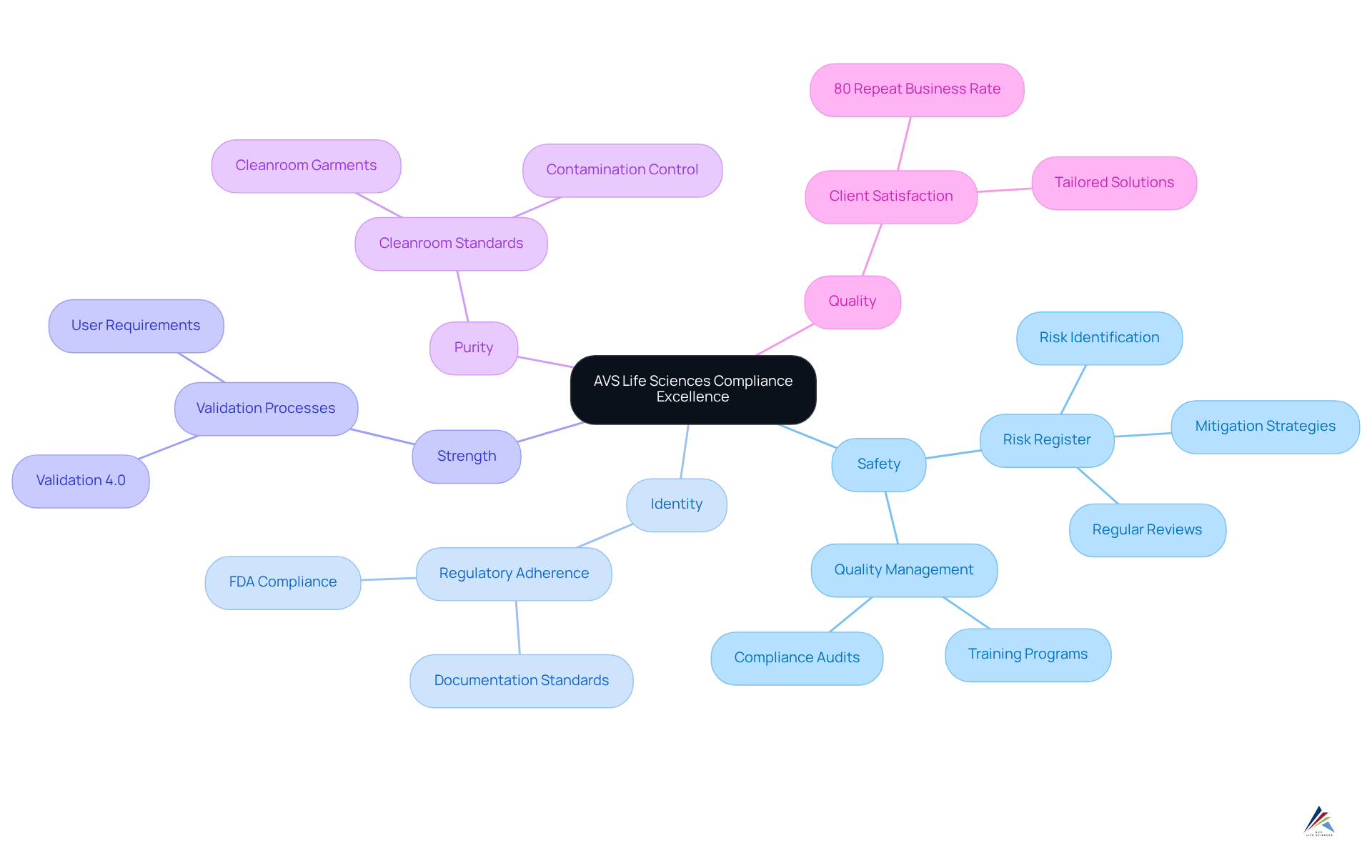
AVS Life Sciences distinguishes itself in the pharmaceutical industry by offering a comprehensive suite of services that ensure compliance with sispq standards, including safety, identity, strength, purity, and quality. With a dedicated team of over 300 seasoned professionals, the company delivers that empower clients to adeptly navigate the complexities of regulatory environments.
By emphasizing quality management and regulatory adherence, AVS Life Sciences enables organizations to uphold rigorous standards, known as sispq, throughout the lifecycle of their offerings. This commitment is underscored by an impressive 80% repeat business rate, reflecting high levels of client satisfaction and trust in their services.
Furthermore, maintaining a risk register is paramount for regulatory adherence and product quality; AVS Life Sciences highlights this practice as a cornerstone of their management strategies for sispq, which encompasses safety, integrity, stability, and quality.
As Jay Tarter, Associate Vice President and Validation Department Manager, aptly states, "Effective management of quality, safety, identity, strength, and purity is essential for enhancing industry performance and fostering confidence in pharmaceutical offerings." This positions AVS Life Sciences as a reliable partner for compliance officers striving for excellence in quality management.
Understanding SISPQ: Definition and Importance in Pharmaceutical Compliance
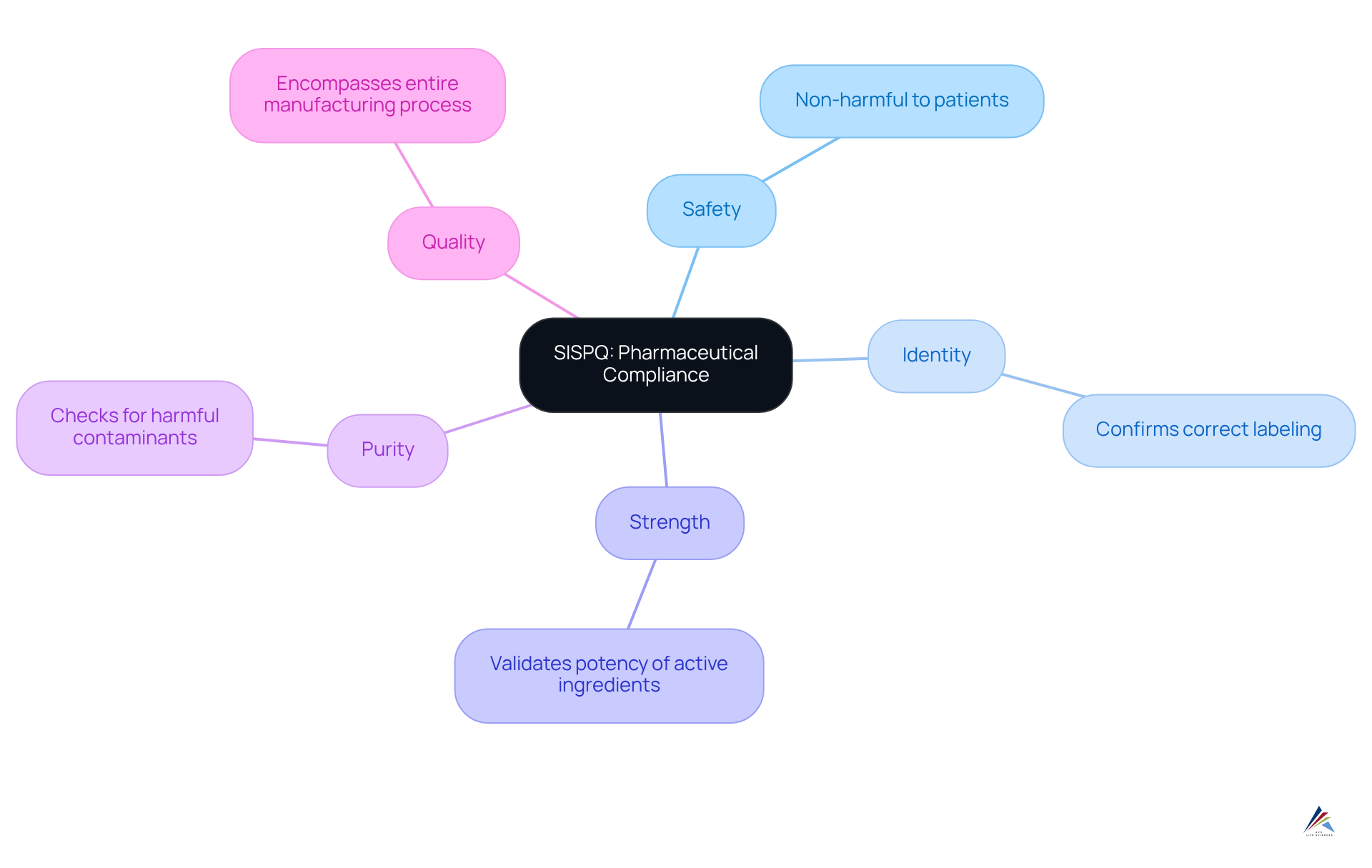
The sispq acronym, which stands for Safety, Identity, Strength, Purity, and Quality, encapsulates essential characteristics that pharmaceutical products must uphold to ensure patient safety and adhere to regulatory standards. Each element is critical:
- Safety guarantees that products are non-harmful to patients;
- Identity confirms that the product corresponds with its labeling;
- Strength validates the potency of active ingredients;
- Purity assesses the absence of harmful contaminants;
- Quality encompasses the entire manufacturing process.
For regulatory officers, prioritizing sispq—quality, safety, identity, strength, and purity—is paramount, as it significantly mitigates risks and preserves the integrity of pharmaceutical offerings.
Shortcomings in compliance with these standards can have dire consequences for patient safety. Historical cases illustrate that lapses in purity can lead to contamination, resulting in severe health issues for patients. Statistics reveal that correlates with increased adverse drug reactions, highlighting the necessity for rigorous adherence to these principles. Regulatory specialists emphasize that maintaining high quality standards, which is essential for sispq, is not merely a regulatory requirement but a fundamental obligation to protect public health.
As Brad Henry, Vice President of Services Products at AVS Life Sciences, remarks, "Ensuring compliance with quality standards is essential not only for regulatory adherence but also for protecting patient health and trust in pharmaceutical offerings." Furthermore, AVS Life Sciences demonstrates its commitment to quality through the effective management of compliance-driven projects, establishing a reputation for excellence within the industry.
Key Elements of SISPQ: Ensuring Quality and Compliance in Pharmaceuticals
The key elements of SISPQ are critical for ensuring compliance and product integrity:
- Safety: It is imperative to ensure that products are devoid of harmful effects.
- Identity: Verification of the item against its labeling and specifications is essential for SISPQ.
- Strength: Confirming that the item contains the precise dosage of active components is crucial for SISPQ.
- Purity: A thorough evaluation for impurities must be conducted to ensure adherence to SISPQ standards.
- Quality: This encompasses every aspect of the manufacturing process, particularly compliance with Good Manufacturing Practices (GMP) while ensuring SISPQ.
To uphold these elements, compliance personnel must implement .

SISPQ and Risk Management: Strategies for Compliance Officers
Compliance officers can significantly enhance SISPQ management by implementing robust risk management strategies, which include:
- Risk Assessment: Conduct regular evaluations of potential risks related to product safety and compliance. This proactive approach is essential, as studies indicate that effective risk assessment can reduce the likelihood of expensive recalls, averaging around 10 million dollars per incident. As Wayne Back mentions, 'The average expense of a pharmaceutical recall is roughly 10 million dollars.'
- Preventive Measures: Establish proactive measures to mitigate identified risks before they adversely affect product quality. For instance, utilizing validation specialists can result in a scientifically robust validation approach, minimizing process failures and improving adherence. Parveen Bhandola, PhD, emphasizes, "It’s better to spend the time and money to develop a scientifically sound validation approach."
- Monitoring and Reporting: Create systems for ongoing observation of quality attributes, ensuring that any deviations are reported without delay. This continuous watchfulness is essential for upholding high standards of product quality and regulatory adherence. Maintaining a detailed risk register is vital, as Wayne Back states, "A risk register is a living document, similar to a Trace Matrix, used in pharmaceutical facilities as a risk management tool."
- Training: Provide comprehensive training for all staff on risk management practices associated with the relevant quality standards. This promotes a culture of adherence and ensures that employees are equipped to identify and address potential risks effectively. Training and communication are essential for staff on the use and .
Recent developments in SISPQ risk assessment emphasize the importance of maintaining a detailed risk register in SISPQ, which serves as a living document to track risks and mitigation strategies. By incorporating these strategies, regulatory personnel can not only fulfill legal obligations but also promote ongoing enhancement within their organizations. A practical suggestion for regulatory professionals is to plan frequent assessments of the risk register to guarantee it stays up-to-date and efficient.

Documentation Practices for SISPQ: Maintaining Compliance and Audit Trails
To maintain compliance and ensure audit readiness, compliance officers must adopt that address the challenges of regulatory requirements.
It is imperative to develop and maintain Standard Operating Procedures (SOPs) for all processes affecting sispq, ensuring consistency and adherence to best practices.
- Batch Records: Detailed batch records must be kept, documenting every step of the manufacturing process to provide a clear trail of compliance.
- Change Control: Establishing a robust change control system is crucial to record any alterations to processes or items that may affect quality, safety, identity, strength, and purity.
- Audit Trails: All electronic records should have secure audit trails to effectively track changes and access, reinforcing accountability and transparency.
By implementing these documentation practices, compliance officers can not only enhance their audit readiness but also foster a culture of compliance that prioritizes quality and safety.

Regulatory Frameworks Impacting SISPQ: A Guide for Compliance Officers
Compliance officers must navigate a complex landscape of regulatory frameworks that significantly influence sispq, which stands for Safety, Identity, Strength, Purity, and Quality. The challenges of maintaining compliance in this environment necessitate a robust understanding of key components, which include:
- Good Manufacturing Practices (GMP): These regulations ensure that products are consistently produced and controlled according to established quality standards. Adhering to GMP is essential, as it directly affects the safety and efficacy of the item. Recent statistics suggest that following GMP standards is crucial for sustaining market access and consumer confidence in pharmaceuticals, with studies demonstrating that firms with high GMP compliance rates encounter a 30% decrease in recalls.
- ISO Standards: The International Organization for Standardization (ISO) provides a framework for quality management systems applicable across various sectors, including pharmaceuticals. Applying ISO standards assists organizations in streamlining processes, enhancing quality, and improving customer satisfaction. For instance, ISO 9001 is widely adopted for quality management, while ISO 13485 focuses specifically on medical devices. A notable example is the implementation of ISO 13485 by a leading pharmaceutical company, which resulted in a 25% increase in operational efficiency and a significant reduction in compliance-related issues.
- Quality System Regulations (QSR): These regulations oversee the quality systems for medical devices and pharmaceuticals, ensuring that items meet safety and effectiveness requirements. Compliance with QSR is mandatory for manufacturers and is closely monitored by regulatory bodies.
- FDA Guidelines: The U.S. Food and Drug Administration (FDA) issues specific guidelines that outline expectations for sispq in the context of drug manufacturing. These recommendations are essential for regulatory agents to grasp, as they determine the criteria for development, testing, and approval procedures. Compliance personnel have observed that understanding these guidelines is crucial for reducing regulatory risks and ensuring successful launches.
Staying informed about the latest advancements in GMP, ISO, QSR, and FDA regulations is essential for quality managers to effectively oversee quality assurance and regulatory adherence within their organizations. This knowledge not only assists in upholding regulations but also improves the overall , ultimately resulting in enhanced patient safety and product reliability.

Training and Development for SISPQ: Empowering Compliance Officers
To empower compliance officers in managing SISPQ, organizations must prioritize the following strategies:
-
Regular Training Programs: Implement ongoing training sessions that cover SISPQ principles, regulatory updates, and industry best practices. Pharma employees should receive training at least annually to ensure they remain knowledgeable about evolving standards and can effectively navigate complex regulatory landscapes.
Encourage involvement in specialized workshops and seminars that are focused on SISPQ and regulatory standards. These events offer significant networking opportunities and insights from industry specialists, thereby enhancing the skills and knowledge of regulatory professionals.
-
Mentorship Programs: Establish mentorship initiatives that connect less experienced regulatory staff with seasoned professionals. This fosters an environment of continuous education and enables the exchange of valuable insights and strategies that can enhance management practices.
-
Access to Resources: Ensure that oversight personnel have access to current resources, including regulatory guidelines, industry publications, and training materials. This empowers them to stay informed and make well-informed decisions in their roles.
-
Best Practices for Training and Development: Adopt a structured approach to training that includes clear objectives, evaluation metrics, and feedback mechanisms. This not only boosts the effectiveness of training programs but also aligns them with organizational objectives, ultimately enhancing compliance personnel performance and improving organizational compliance outcomes.

Challenges in Implementing SISPQ: Solutions for Compliance Officers
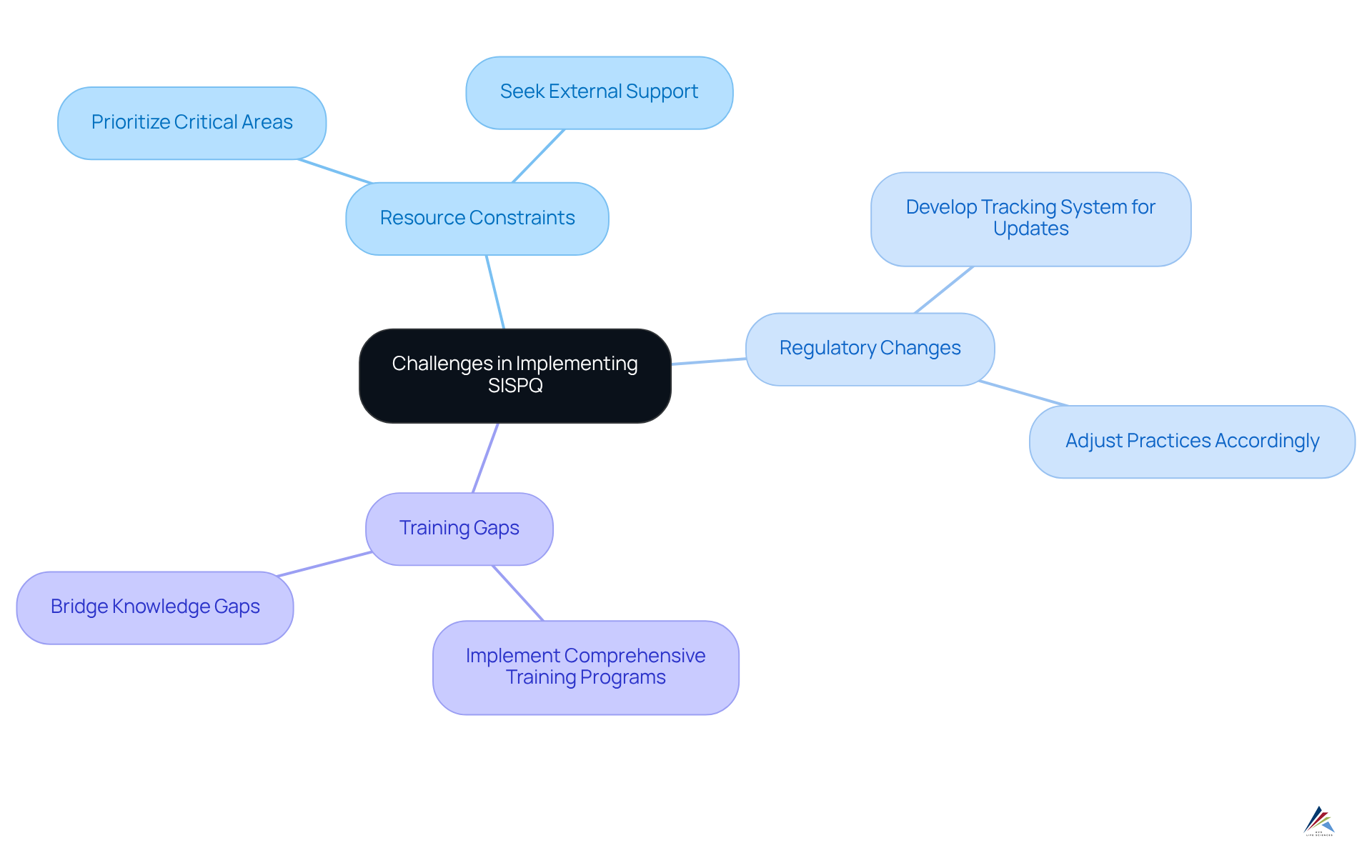
Compliance officers encounter several significant challenges when implementing sispq protocols.
- Resource constraints pose a critical issue, as limited resources can impede the ability to uphold sispq. To address this, prioritizing critical areas and seeking external support when necessary can be .
- Furthermore, regulatory changes related to sispq present a daunting task; keeping abreast of evolving regulations is essential. Compliance personnel should develop a robust system for tracking regulatory updates and adjusting practices accordingly.
- Lastly, training gaps identified as sispq can lead to regulatory failures due to inadequate training. Organizations must prioritize comprehensive training programs to effectively bridge these knowledge gaps.
By proactively addressing these challenges, compliance officers can foster a more resilient and compliant operational framework.
Continuous Improvement in SISPQ: Best Practices for Compliance Officers
To foster continuous improvement in sispq compliance, compliance officers must implement best practices that address key challenges related to sispq in the field.
Regular audits are essential for evaluating adherence to the sispq standards. These audits not only help recognize adherence gaps but also identify areas needing improvement, ensuring that organizations remain aligned with regulatory expectations. As Paul Koziarz aptly states, "You have to assess adherence not as a cost, but as a money saver."
- Feedback Mechanisms: Establishing robust channels for feedback from staff and stakeholders is crucial. This input can reveal potential issues and highlight opportunities for improvement, fostering a culture of open communication and proactive problem-solving. Bill Harrison emphasizes that "whether you need to navigate a possible security breach or a potential audit, it is beneficial to be ready in advance with exceptional adherence protocols."
- Benchmarking: Comparing practices related to sispq against industry standards and best practices allows organizations to pinpoint deficiencies and identify areas for growth. This benchmarking process is vital for sustaining competitive adherence and operational excellence.
- Action Plans: Developing and implementing action plans based on audit findings and stakeholder feedback is key to driving continuous improvement. These plans should outline specific steps to address identified issues, ensuring that adherence efforts are both strategic and effective. Utilizing frameworks such as the Plan-Do-Check-Act (PDCA) cycle can provide a structured approach to this process, reinforcing the commitment to .

Future Trends in SISPQ: Preparing Compliance Officers for Change
Compliance officers must remain vigilant regarding emerging trends that significantly impact SISPQ management, including:
- Digital Transformation: The adoption of digital tools and technologies in manufacturing processes is revolutionizing SISPQ management. These advancements can enhance efficiency and conformity; however, they necessitate rigorous oversight to ensure adherence to regulatory standards. An astonishing 97% of firms indicated that the COVID-19 pandemic accelerated their digital transformation initiatives, underscoring the necessity for regulatory professionals to embrace these changes. AVS Life Sciences, with its extensive experience and tailored consulting expertise, is well-positioned to assist organizations in navigating these transformations effectively.
- Regulatory Evolution: As regulations continuously change, it is essential for oversight officers to stay informed and adaptable in modifying their practices. This adaptability is crucial for meeting new requirements and ensuring ongoing compliance in a dynamic regulatory landscape. AVS Life Sciences' proven track record, evidenced by an 80% repeat business rate, highlights its effectiveness in supporting clients through these changes.
- Sustainability Initiatives: The growing emphasis on sustainability is transforming practices within organizations. Compliance personnel are now tasked with integrating environmental factors into their strategies, aligning with broader industry movements towards sustainable practices. This shift is essential for organizations aiming to .
- Data Analytics: Leveraging data analytics provides valuable insights into performance, enabling regulatory personnel to identify areas for enhancement. This data-focused approach not only improves operational efficiency but also aids in proactive regulation management. As noted by industry leaders, the ability to learn and adapt is the greatest competitive advantage in today's environment.
In this rapidly changing landscape, organizations must prioritize digital transformation as a fundamental aspect of their operational strategy to thrive in the future. As Katherine Kostereva, CEO of Creatio, states, "Digital transformation is not a project—it’s a company philosophy." This mindset is crucial for compliance officers as they navigate the complexities of SISPQ.

Conclusion
AVS Life Sciences emphasizes the paramount importance of SISPQ—Safety, Identity, Strength, Purity, and Quality—in ensuring compliance and safeguarding public health within the pharmaceutical industry. By underscoring the necessity for rigorous adherence to these standards, this article highlights the vital role of compliance officers in upholding these essential principles, thereby mitigating risks and enhancing product integrity.
The examination of key elements of SISPQ, alongside risk management strategies, documentation practices, and the regulatory frameworks impacting compliance, offers a thorough overview of the challenges and best practices that compliance officers must navigate. Insights into training and development further stress the need for continuous education and adaptation in an ever-evolving regulatory landscape.
As the industry encounters ongoing changes driven by digital transformation, regulatory evolution, and sustainability initiatives, it is imperative for compliance officers to remain informed and proactive. Embracing these trends will not only bolster compliance efforts but also cultivate a culture of excellence that prioritizes patient safety and product reliability. The commitment to SISPQ transcends mere regulatory compliance; it embodies the pursuit of the highest standards in pharmaceutical practices for the well-being of all.
Frequently Asked Questions
What does SISPQ stand for in the pharmaceutical industry?
SISPQ stands for Safety, Identity, Strength, Purity, and Quality, which are essential characteristics that pharmaceutical products must uphold to ensure patient safety and comply with regulatory standards.
Why is SISPQ important for pharmaceutical compliance?
SISPQ is crucial because it mitigates risks and preserves the integrity of pharmaceutical offerings. Non-compliance with these standards can lead to severe consequences for patient safety, including contamination and adverse drug reactions.
What are the key elements of SISPQ?
The key elements of SISPQ include: - Safety: Ensuring products are non-harmful. - Identity: Confirming the product matches its labeling. - Strength: Validating the potency of active ingredients. - Purity: Assessing the absence of harmful contaminants. - Quality: Encompassing the entire manufacturing process, especially compliance with Good Manufacturing Practices (GMP).
How does AVS Life Sciences support compliance with SISPQ standards?
AVS Life Sciences offers a comprehensive suite of services that ensure compliance with SISPQ standards, emphasizing quality management and regulatory adherence. They provide customized solutions and maintain a risk register as part of their management strategies.
What is the significance of maintaining a risk register in SISPQ compliance?
Maintaining a risk register is vital for regulatory adherence and product quality, allowing organizations to identify and mitigate potential risks associated with safety, integrity, stability, and quality.
What is the impact of AVS Life Sciences' commitment to quality on their business?
AVS Life Sciences has an impressive 80% repeat business rate, indicating high levels of client satisfaction and trust in their services, which reflects their commitment to quality and compliance.
What are the consequences of failing to comply with SISPQ standards?
Shortcomings in compliance with SISPQ standards can lead to contamination and severe health issues for patients, as well as increased adverse drug reactions, underscoring the necessity for rigorous adherence to these principles.
