10 Essential GxP Regulations for Pharmaceutical Compliance

Overview
The article emphasizes the critical GxP (Good Practice) regulations that pharmaceutical companies must adhere to in order to ensure the quality, safety, and efficacy of their products. Compliance with these regulations—including Good Manufacturing Practices (GMP), Good Laboratory Practices (GLP), and Good Clinical Practices (GCP)—is essential for maintaining public trust and protecting patient health. This necessity is underscored by the notable rise in drug recalls and the expanding market for compliance solutions. As such, understanding and implementing these practices is not merely a regulatory requirement but a vital component of operational integrity in the pharmaceutical industry.
Introduction
The pharmaceutical industry operates within a complex web of regulations that are meticulously designed to ensure safety, efficacy, and quality in drug development and manufacturing. As these regulations continue to evolve, understanding the essential Good Practice (GxP) regulations becomes imperative for companies striving to maintain compliance while fostering innovation. This article explores ten critical GxP regulations that not only shape the operational frameworks of pharmaceutical companies but also bolster their credibility and enhance consumer trust.
How can organizations effectively navigate these regulations to mitigate risks and ensure successful product outcomes in an increasingly competitive market?
AVS Life Sciences: Comprehensive GxP Compliance Solutions for Pharmaceutical Companies
AVS Life Sciences provides an extensive array of GxP regulations solutions specifically designed for drug companies. By prioritizing quality management and regulatory compliance, AVS enables clients to navigate the complexities of the drug industry with efficiency. Their offerings include:
- Validation
- Quality assurance consulting
- Engineering support
These are essential elements for meeting stringent regulatory requirements throughout the product lifecycle. This comprehensive approach not only enhances adherence but also promotes innovation and operational efficiency within drug companies.
As the GxP regulation testing market is projected to grow from approximately USD 13.98 billion in 2023 to USD 20.3 billion by 2031, with a compound annual growth rate (CAGR) of 10.3% from 2024 to 2031, the demand for robust adherence solutions is more critical than ever. The U.S. drug industry, valued at over USD 500 billion, drives the need for regulatory testing services, perfectly aligning with AVS Life Sciences' offerings. Furthermore, North America is expected to lead the medication adherence software market, with an estimated market size of around USD 600 million in 2023 and a CAGR of 7.5% from 2024 to 2032.
AVS Life Sciences sets itself apart by offering phase-appropriate quality strategies and comprehensive training programs, specifically designed to aid organizations in managing the complexities of regulatory requirements. Given the increasing intricacy of drug manufacturing processes and heightened scrutiny from regulatory bodies, AVS Life Sciences is committed to equipping clients with the necessary tools and expertise to achieve successful GxP regulations compliance. This dedication not only positions AVS as a in the life sciences arena but also enhances the overall reliability and effectiveness of medical offerings.
Good Manufacturing Practices (GMP): Ensuring Quality and Safety in Pharmaceuticals
Good Manufacturing Practices (GMP) are essential guidelines that safeguard the quality and safety of medicinal items. Covering every phase of production—from sourcing raw materials to delivering the final product—these guidelines ensure that manufacturers adhere to stringent quality standards. By following GMP, risks associated with pharmaceutical manufacturing are significantly reduced, thereby enhancing consumer confidence in the products offered.
Notably, the number of drug recalls surged from approximately 300 in fiscal year 2019 to nearly 800 in fiscal year 2021, underscoring the critical necessity of GMP compliance in maintaining product quality and security. Organizations that have successfully implemented GMP standards report heightened consumer trust, a vital asset in a market where safety is paramount.
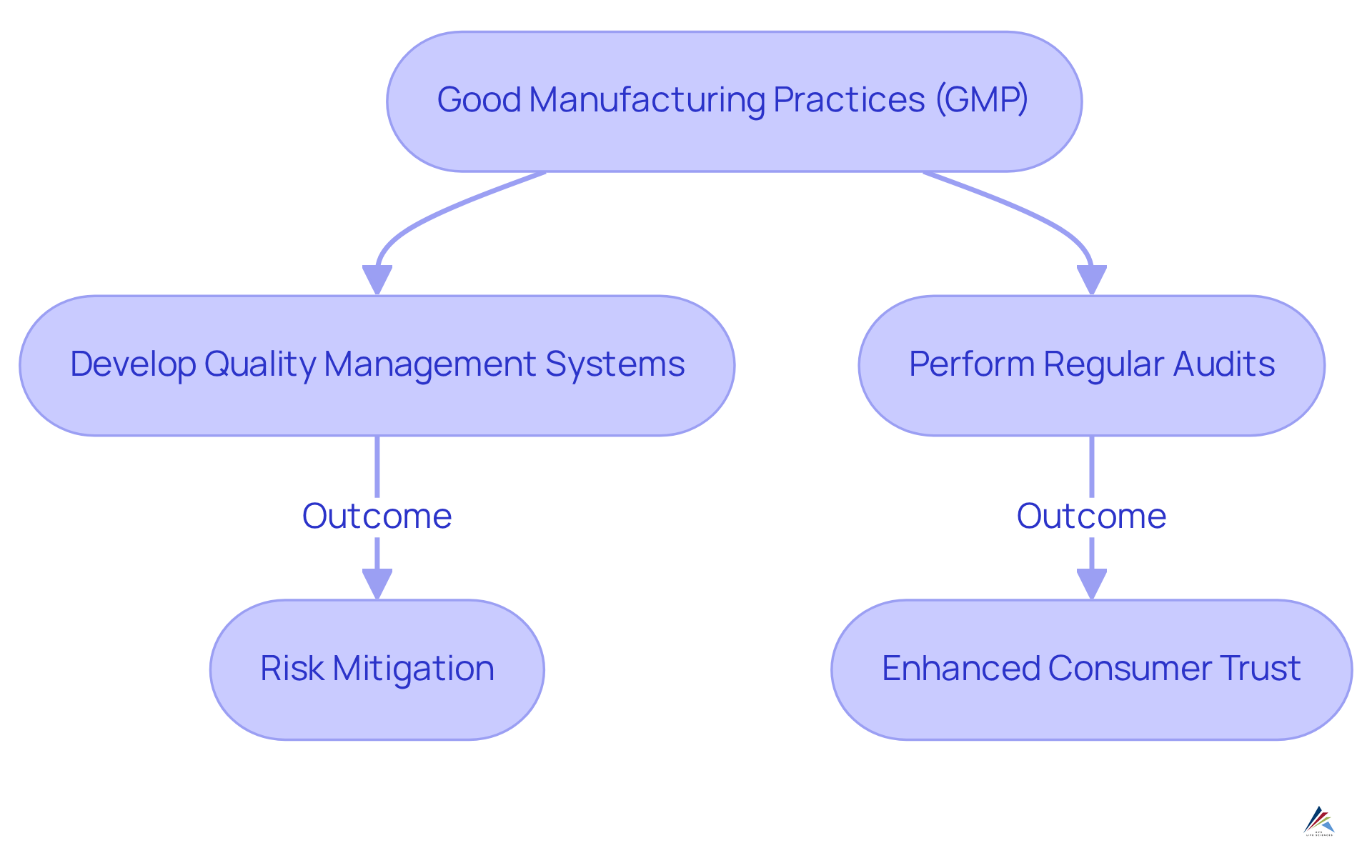
To achieve and sustain GMP standards, companies must:
- Develop robust quality management systems
- Perform regular audits
This reinforces their dedication to quality and safety. With the projected to reach $28 billion by 2033, the demand for [compliance with GxP regulations](https://avslifesciences.com/industry-news/automation-validation-solutions-llc-acquires-mwa-consulting-inc) is more pertinent than ever. This proactive approach not only mitigates compliance risks but also positions companies as trustworthy players in the industry.
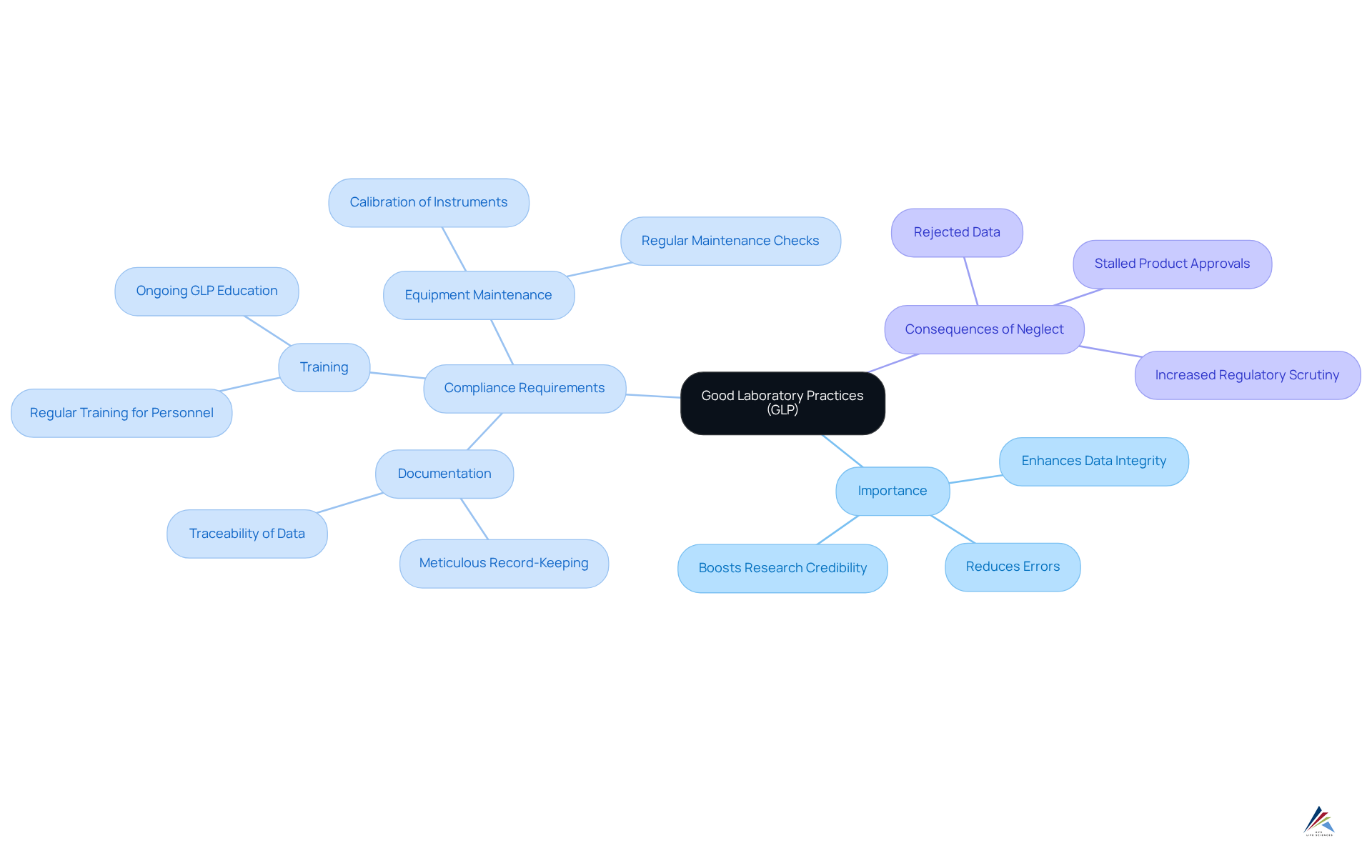
Good Laboratory Practices (GLP): Maintaining Data Integrity in Pharmaceutical Research
Good Laboratory Practices (GLP) are essential guidelines that uphold the quality and integrity of data generated in laboratory studies, particularly within the pharmaceutical sector. These practices establish a robust framework for conducting research that yields .
Compliance with GxP regulations requires:
- Meticulous documentation
- Regular training for laboratory personnel
- Calibration and maintenance of all equipment
Organizations that adhere to GLP not only bolster their regulatory submissions but also significantly enhance the credibility of their research findings. The significance of GLP is underscored by its role in reducing errors and enhancing data integrity, which is crucial for successful development and compliance with GxP regulations.
The 2020 OECD Position Paper highlights the influence of sponsors on GLP study conclusions, emphasizing the necessity for strict adherence to GLP to maintain research credibility. Furthermore, GxP regulations, particularly 21 CFR Part 211 and Part 58, detail the legal ramifications of GLP adherence.
As the pharmaceutical landscape evolves, upholding GLP compliance remains a fundamental aspect of guaranteeing the reliability and effectiveness of medicinal offerings. Neglecting GLP can lead to rejected data and stalled product approvals, reinforcing the critical need for these practices.
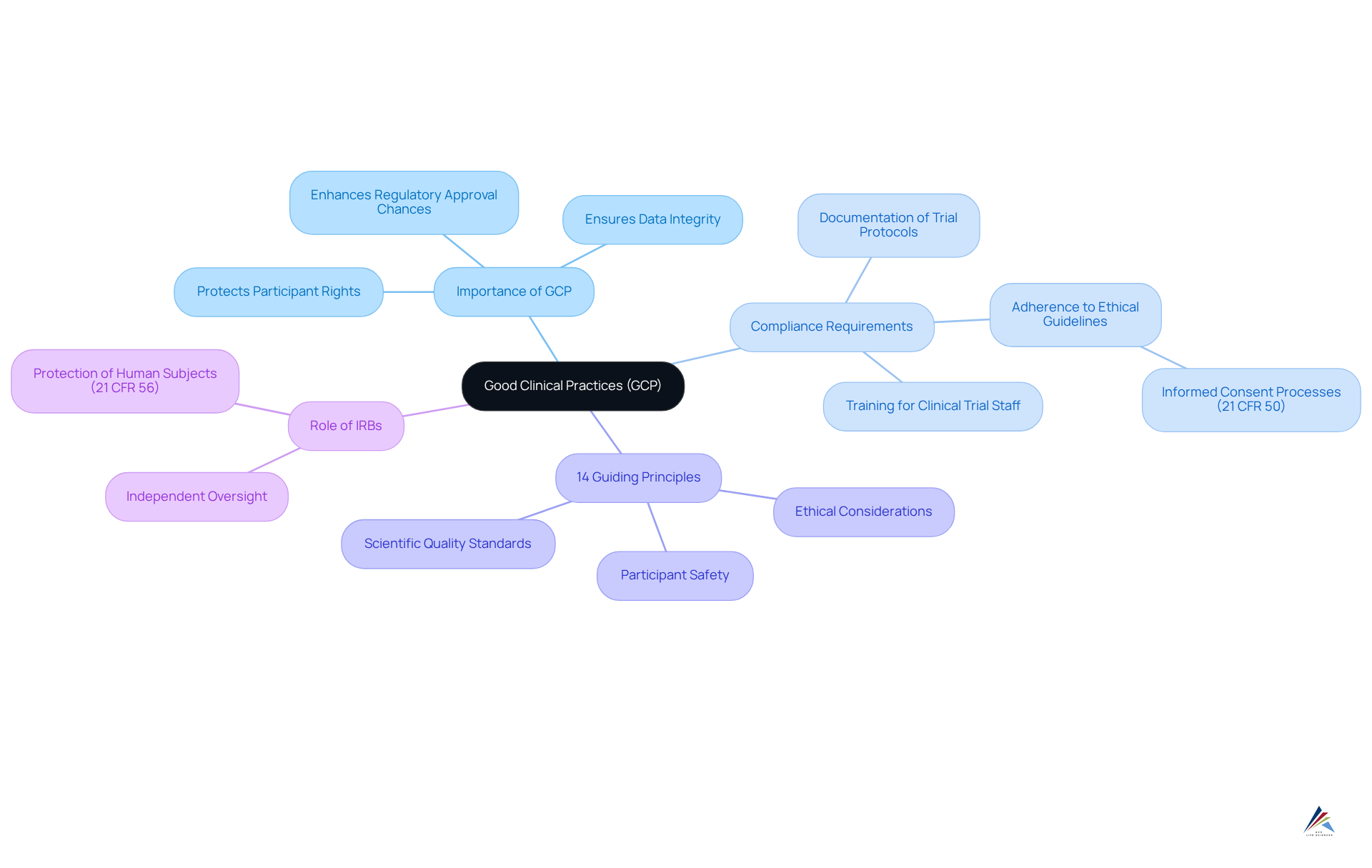
Good Clinical Practices (GCP): Upholding Ethical Standards in Clinical Trials
Good Clinical Practices (GCP) embody a critical set of international ethical and scientific quality standards that are vital for the design, conduct, recording, and reporting of clinical trials. By prioritizing GCP, organizations not only protect the rights, security, and well-being of trial participants but also uphold the integrity of clinical data.
Compliance with GCP requires:
- Comprehensive training for clinical trial staff
- Meticulous documentation of trial protocols
- Strict adherence to ethical guidelines, including the necessity for informed consent processes as outlined in 21 CFR 50
This unwavering commitment to GCP is essential for maintaining public trust, as it directly influences the and the likelihood of successful regulatory approvals. Notably, organizations that consistently implement GCP principles, including the 14 guiding principles of GCP, have demonstrated enhanced participant safety and improved trial integrity, reinforcing the necessity of these standards in the clinical research landscape.
Furthermore, the role of Institutional Review Boards (IRBs), as defined in 21 CFR 56, is crucial for providing independent oversight to protect trial participants, further underscoring the importance of GCP compliance.
Quality System Regulations (QSR): Framework for Quality Management in Medical Devices
Quality System Regulations (QSR) serve as a critical framework for quality management within the medical device sector. They ensure that manufacturers develop and maintain effective quality systems throughout the entire lifecycle—from design and development to production and post-market surveillance.
Compliance with QSR necessitates the implementation of standard operating procedures, regular audits, and meticulous documentation practices. This regulatory adherence is vital, not only for ensuring the but also for meeting stringent regulatory requirements.
Organizations that successfully apply QSR can significantly enhance their lifecycle management, leading to improved operational efficiency and reduced time to market. For instance, companies that have integrated QSR principles into their processes have reported fewer compliance-related issues and a stronger market presence.
As the medical device landscape continues to evolve, prioritizing QSR compliance remains essential for manufacturers striving to deliver high-quality offerings while navigating complex regulatory environments.
Good Distribution Practices (GDP): Safeguarding Pharmaceutical Product Integrity
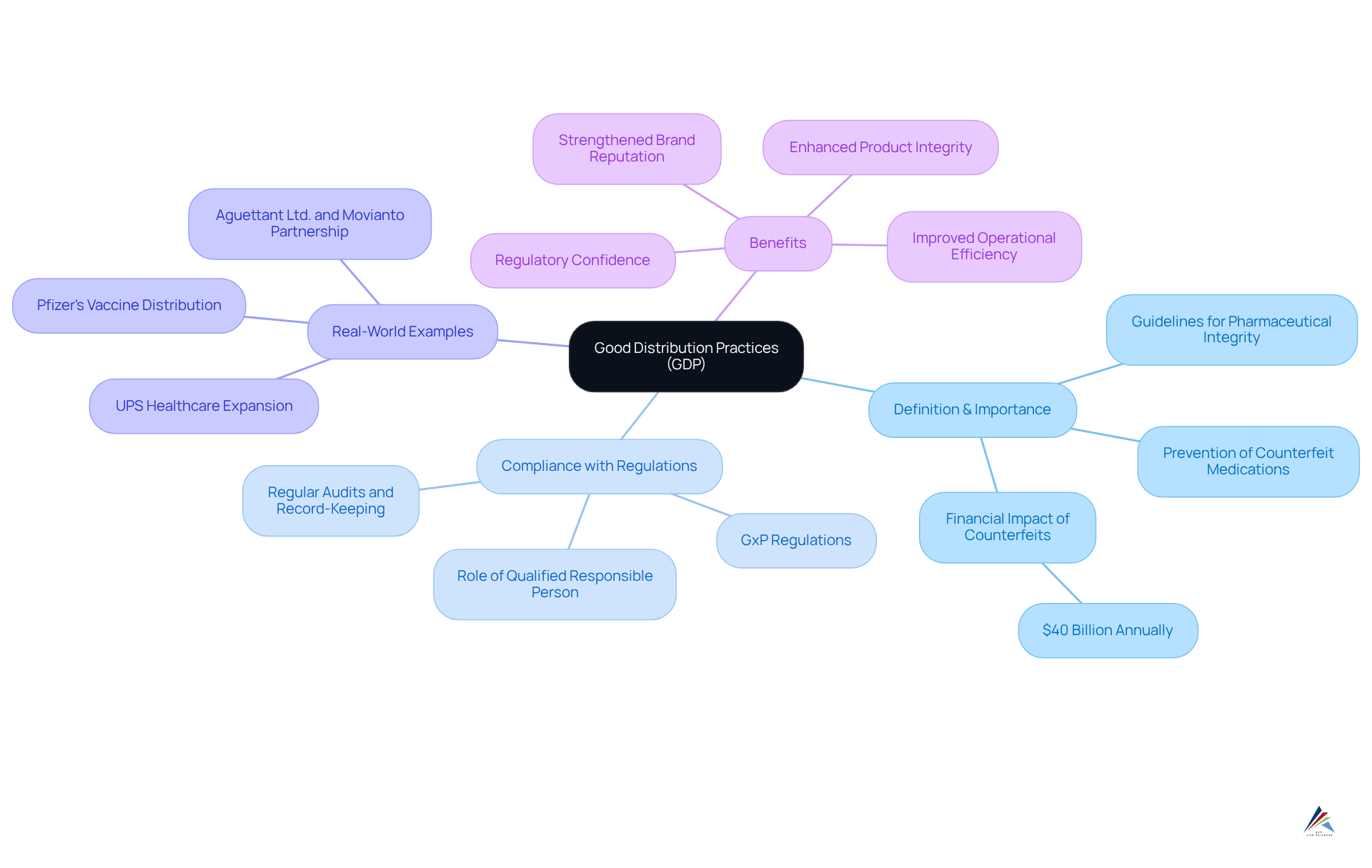
Good Distribution Practices (GDP) are essential guidelines that uphold the integrity and quality of pharmaceutical items throughout the distribution process. By encompassing every aspect of the supply chain—from storage and transportation to handling and documentation—GDP guarantees that items remain safe and effective for patients. Compliance with GxP regulations necessitates the implementation of appropriate storage conditions, meticulous record-keeping, and regular audits of distribution practices. This rigorous adherence is vital in preventing deterioration and mitigating risks associated with , which can have dire consequences for patient safety and result in an estimated financial impact of approximately $40 billion annually due to counterfeit drugs.
The significance of GDP is underscored by its capacity to enhance product integrity during distribution. For instance, GDP facilitates rapid trace-and-track capabilities, enabling organizations to pull suspect lots within hours, thereby safeguarding public health. Moreover, the integration of advanced technologies, such as IoT devices and blockchain, is revolutionizing adherence to GxP regulations by improving visibility and traceability throughout the supply chain. These innovations not only optimize operations but also bolster compliance with GxP regulations, as evidenced by the increasing emphasis on digital transformation within GDP frameworks.
Real-world examples further illustrate the effectiveness of GDP in ensuring the integrity of goods. The collaboration between Aguettant Ltd. and Movianto exemplifies how adherence to GDP, including a documented quality system covering all outsourced lanes, can ensure a reliable supply of infusion solutions to NHS hospitals, thereby enhancing patient care. Similarly, Pfizer's use of GPS-enabled thermal sensors in the distribution of COVID-19 vaccines demonstrates how stringent GDP adherence can meet essential storage requirements, ensuring the secure transport of life-saving items.
As the healthcare landscape evolves, the importance of GDP in ensuring the safety and effectiveness of items cannot be overstated. Organizations that prioritize GDP adherence not only protect their brand reputation but also foster stakeholder trust, ultimately positioning themselves as leaders in the competitive medicine market. The benefits of adhering to GDP, which include improved item quality, regulatory confidence, and a strengthened brand reputation, are crucial for success in today's complex regulatory environment, especially with GxP regulations in place.
Good Agricultural Practices (GAP): Ensuring Safety and Quality of Raw Materials
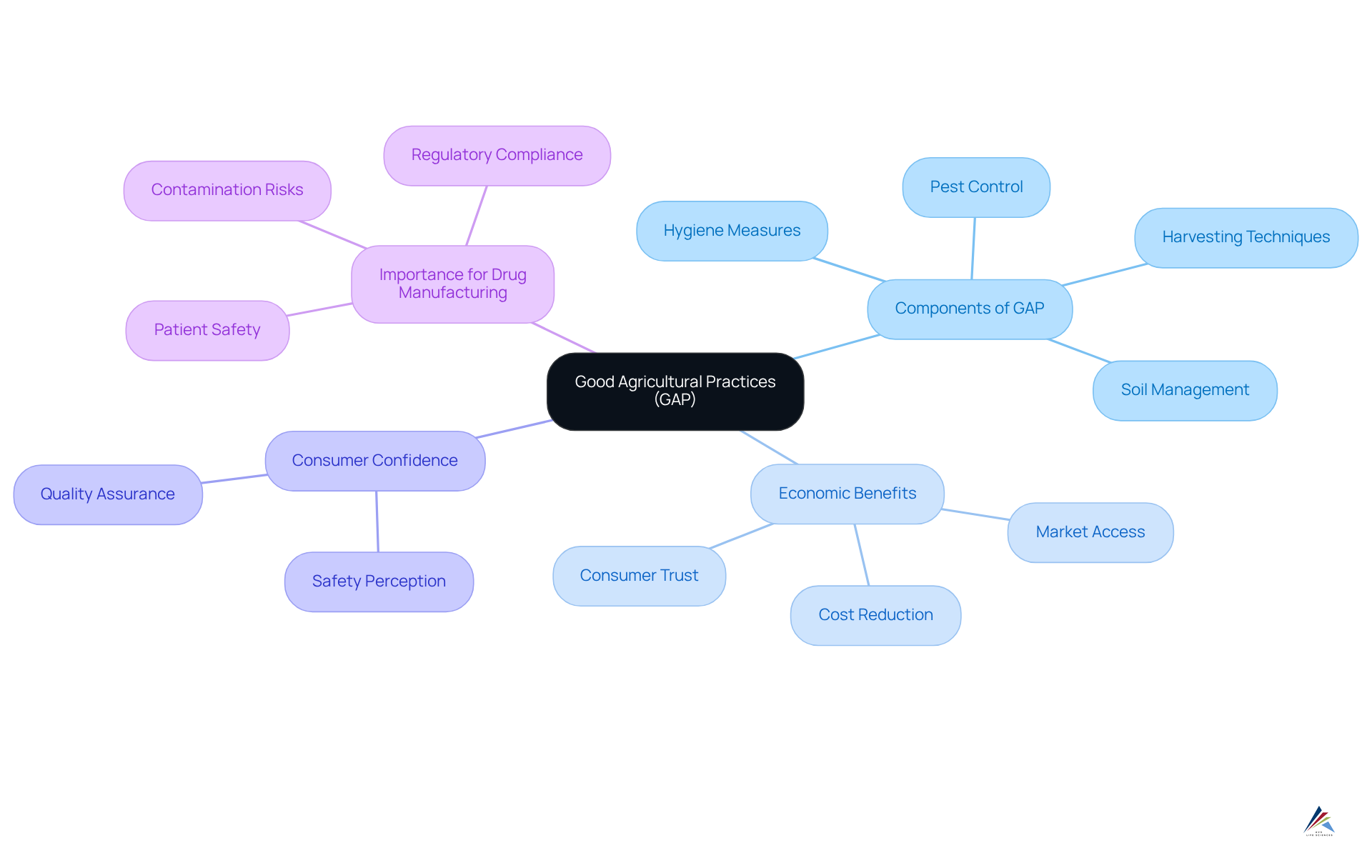
Good Agricultural Practices (GAP) are essential guidelines that safeguard the integrity and quality of raw materials utilized in drug manufacturing. These practices encompass various elements of agricultural production, such as soil management, pest control, and harvesting techniques. By adhering to GAP, organizations can significantly mitigate contamination risks, ensuring that raw materials consistently meet stringent quality standards. Notably, the implementation of GAP has been demonstrated to enhance crop quality while reducing environmental impact, which is vital for maintaining the integrity of pharmaceutical products.
As of July 2025, it is increasingly recognized that adherence to GAP transcends regulatory obligation, emerging as a critical component of raw material security. Approximately 60% of drug recalls are linked to raw material quality issues, emphasizing the necessity for robust GAP adherence. A case study on the adoption of GAPs in agricultural practices illustrated that a family-owned farm in California accessed premium markets by implementing these standards, showcasing the economic advantages of compliance. Furthermore, certification programs for GAPs can lead to improved market access and heightened consumer trust, further underscoring the economic benefits of adhering to these practices.
Moreover, utilizing GAP can bolster consumer confidence in the reliability and quality of agricultural goods, an essential factor for the success of the pharmaceutical sector. As John Patel, a Food Safety Consultant, asserts, "GAPs act as the foundation for establishing consumer confidence in the security and quality of agricultural goods." Complementing this, Dr. Maria Lopez, an Agricultural Scientist, emphasizes, "The adoption of Good Agricultural Practices is crucial in guaranteeing the security and quality of our food supply." This trust is indispensable for drug companies that rely on high-quality raw materials to ensure patient safety and product efficacy. Thus, emphasizing GAP adherence is vital for drug companies aiming to and meet GxP regulations. Additionally, thorough planning, monitoring, and record-keeping are fundamental components of effective GAP implementation, ensuring compliance with established standards.
Good Pharmacovigilance Practices (GPvP): Monitoring Post-Marketing Safety of Pharmaceuticals
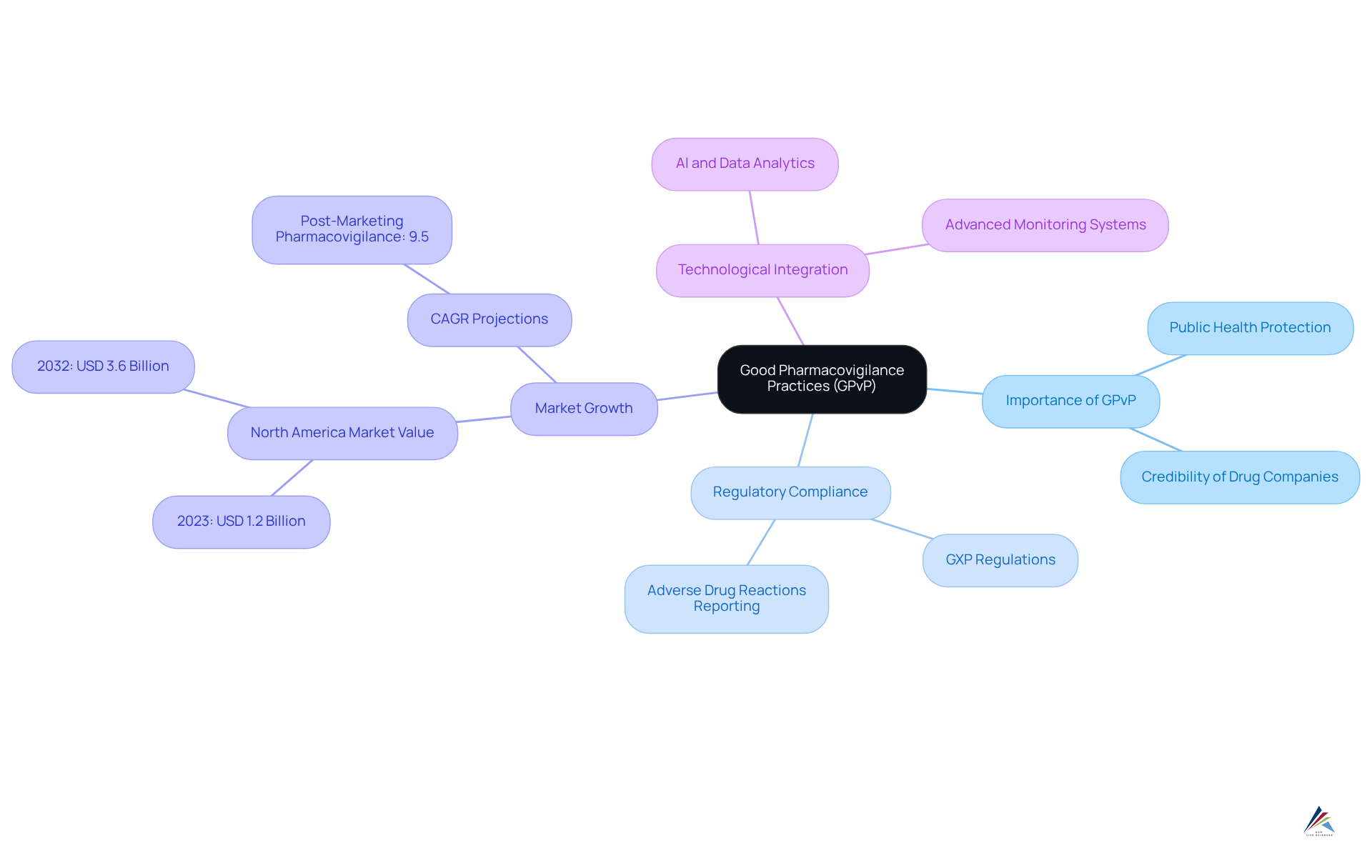
Good Pharmacovigilance Practices (GPvP) are essential for guaranteeing the wellbeing of drugs once they enter the market. This framework involves the systematic collection and analysis of data concerning adverse events and product performance, which is crucial for identifying potential risks associated with medications. Adherence to GPvP not only protects public health but also boosts the credibility of drug companies by demonstrating their dedication to patient welfare.
Organizations are required to implement robust pharmacovigilance systems that align with GXP regulations to maintain regulatory approval. This includes establishing for adverse drug reactions (ADRs) and conducting thorough risk assessments. For instance, the North America Pharmacovigilance Market was valued at USD 1.2 billion in 2023 and is expected to expand considerably, reaching USD 3.6 billion by 2032, fueled by the rising occurrence of ADRs and the demand for efficient monitoring.
Several organizations have successfully integrated GPvP into their risk management strategies. Prominent drug companies are utilizing advanced data analytics and AI technologies to enhance their pharmacovigilance capabilities, ensuring prompt identification and mitigation of risk issues. The emphasis on GPvP is particularly relevant as the market evolves, especially in the context of GXP regulations, with a projected compound annual growth rate (CAGR) of 9.5% for the post-marketing pharmacovigilance and medical information market from 2024 to 2030.
In July 2025, the significance of GPvP will be further underscored as regulatory bodies continue to refine their expectations for post-marketing risk assessments. By adhering to GxP regulations, organizations not only meet regulatory requirements but also enhance the overall security and effectiveness of pharmaceuticals in the marketplace, ultimately safeguarding public health. As Rahul Gotadki observed, "The stringent regulations established by the Food and Drug Administration (FDA) and other oversight bodies, which require continuous drug monitoring, are one of the primary factors driving the market.
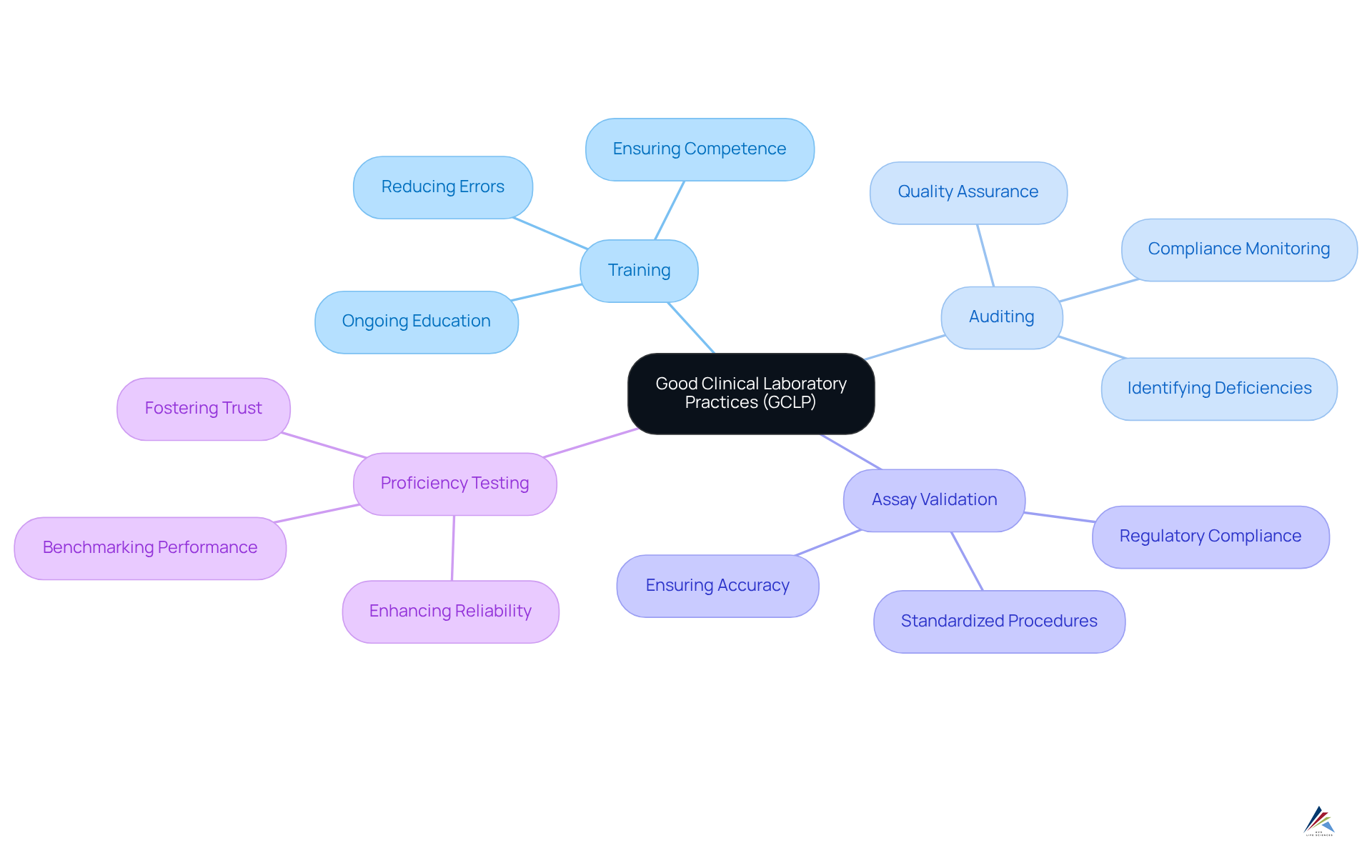
Good Clinical Laboratory Practices (GCLP): Ensuring Quality in Clinical Testing
Good Clinical Laboratory Practices (GCLP) are essential guidelines that uphold the quality and reliability of clinical testing. These practices encompass all facets of laboratory operations, including , equipment maintenance, and data management. Adhering to GCLP is crucial for generating accurate and reproducible test results, which are vital for regulatory submissions and informed clinical decision-making.
Organizations that implement GCLP standards have reported a 35% reduction in errors achieved through training and quality control measures, significantly improving testing accuracy and reliability. Furthermore, a unified set of GCLP-defined procedures enhances the reproducibility of results, especially in trials involving multiple international laboratory sites.
As the worldwide agreement on GCLP adherence strengthens, it is increasingly acknowledged as the minimal requirement for clinical laboratories to optimize their operations and ensure participant safety. The four crucial components of GCLP—training, auditing, assay validation, and proficiency testing—are vital for adherence and should be prioritized by organizations.
The importance of GCLP cannot be overstated; it not only safeguards the integrity of clinical testing processes but also fosters trust in the results produced, ultimately benefiting patient outcomes and advancing the field of medical research.
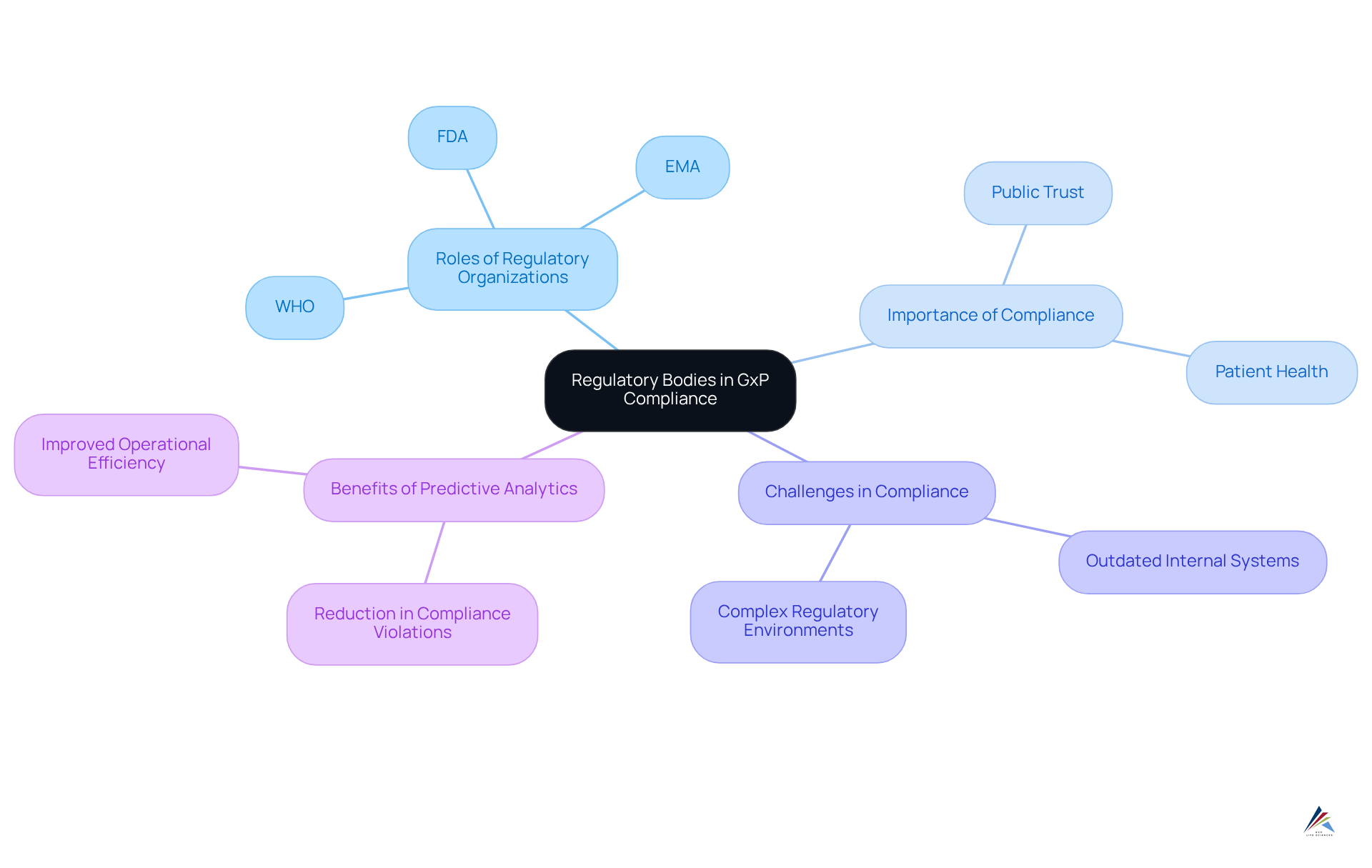
Regulatory Bodies: Enforcing GxP Compliance in the Pharmaceutical Industry
Regulatory organizations such as the FDA, EMA, and WHO play an indispensable role in enforcing gxp regulations within the drug industry. They establish comprehensive guidelines and regulations that govern medical practices, ensuring both safety and effectiveness. Compliance with these regulations transcends mere legal obligation; it is vital for maintaining public trust and safeguarding patient health.
These organizations conduct thorough inspections, audits, and reviews to verify adherence to gxp regulations, which significantly influence a company's operational integrity and market access. Notably, drug manufacturers that adeptly navigate these regulations often experience a 40% reduction in adherence violations through the use of predictive analytics, which enhances efficiency in their processes and improves product quality.
Understanding the is essential for pharmaceutical companies striving to successfully navigate the intricate regulatory landscape. By aligning their practices with established guidelines, organizations can mitigate risks, avoid costly penalties, and cultivate a culture of adherence that supports sustainable growth.
Furthermore, it is noteworthy that 64% of compliance programs encounter challenges due to outdated internal systems, emphasizing the urgent need for current knowledge and practices in compliance. This underscores the importance of integrating regulatory analytics, which has been demonstrated to streamline drug approval processes and bolster operational efficiency.
Conclusion
The significance of GxP regulations in the pharmaceutical industry is paramount, as they form the foundation for ensuring product quality, safety, and efficacy. By adhering to these essential guidelines, pharmaceutical companies not only fulfill legal obligations but also cultivate trust among consumers and stakeholders. The comprehensive approach to GxP compliance, exemplified by organizations like AVS Life Sciences, empowers drug manufacturers to adeptly navigate the complexities of regulatory landscapes.
In this article, we explored key regulations such as:
- Good Manufacturing Practices (GMP)
- Good Laboratory Practices (GLP)
- Good Clinical Practices (GCP)
Each regulation plays a vital role at various stages of the pharmaceutical lifecycle, from sourcing raw materials to conducting post-marketing surveillance. The focus on robust quality management systems, meticulous documentation, and continuous training underscores the proactive measures that companies must embrace to mitigate risks and enhance operational efficiency.
As the pharmaceutical landscape evolves, the necessity for stringent GxP compliance remains critical. Companies must prioritize these regulations not only to avert potential penalties but also to safeguard patient safety and well-being. By fostering a culture of compliance and investing in innovative solutions, organizations can establish themselves as leaders in the industry, ultimately contributing to the advancement of public health and the integrity of pharmaceutical offerings.
Frequently Asked Questions
What services does AVS Life Sciences provide for pharmaceutical companies?
AVS Life Sciences offers a comprehensive array of GxP compliance solutions, including validation, quality assurance consulting, and engineering support, aimed at helping drug companies meet regulatory requirements throughout the product lifecycle.
Why is GxP compliance important for drug companies?
GxP compliance is essential for ensuring quality management and regulatory adherence, which enhances innovation and operational efficiency, ultimately leading to improved reliability and effectiveness of medical offerings.
What is the projected growth of the GxP regulation testing market?
The GxP regulation testing market is projected to grow from approximately USD 13.98 billion in 2023 to USD 20.3 billion by 2031, with a compound annual growth rate (CAGR) of 10.3% from 2024 to 2031.
How do Good Manufacturing Practices (GMP) contribute to pharmaceutical safety?
GMP guidelines ensure that manufacturers adhere to stringent quality standards throughout all phases of production, significantly reducing risks associated with pharmaceutical manufacturing and enhancing consumer confidence in the products.
What must companies do to achieve and maintain GMP standards?
Companies must develop robust quality management systems and perform regular audits to reinforce their dedication to quality and safety.
What are Good Laboratory Practices (GLP) and why are they important?
GLP are essential guidelines that maintain the quality and integrity of data generated in laboratory studies, ensuring reliable and reproducible results, which are crucial for regulatory compliance.
What requirements must organizations meet to comply with GLP regulations?
Organizations must ensure meticulous documentation, provide regular training for laboratory personnel, and maintain calibration and upkeep of all equipment.
What are the consequences of neglecting GLP compliance?
Neglecting GLP can lead to rejected data and stalled product approvals, highlighting the critical need for adherence to these practices to ensure the reliability of medicinal offerings.
