10 Essential GMP Requirements for Compliance Officers in Pharma

Overview
The article addresses the critical GMP (Good Manufacturing Practices) requirements that compliance officers in the pharmaceutical industry must uphold to ensure regulatory compliance and product quality. It underscores the necessity of a robust quality management system, effective training programs, and meticulous documentation practices. These elements are essential for navigating the complex regulatory landscape and maintaining high standards in pharmaceutical manufacturing. By implementing these practices, compliance officers can effectively mitigate risks and enhance product integrity, ultimately fostering a culture of quality and compliance within their organizations.
Introduction
The pharmaceutical industry operates within a complex regulatory framework, where adherence to Good Manufacturing Practices (GMP) is essential for ensuring product quality and patient safety. Compliance officers are pivotal in navigating these challenges, equipped with the necessary knowledge and strategies to uphold stringent standards. As regulatory scrutiny intensifies and the demand for high-quality pharmaceuticals escalates, organizations face a pressing question: how can they effectively implement GMP requirements while overcoming prevalent compliance obstacles? This article explores ten fundamental GMP requirements that compliance officers must master to thrive in the dynamic landscape of pharmaceutical manufacturing.
AVS Life Sciences: Comprehensive Quality Compliance Solutions for GMP
AVS Life Sciences delivers a robust suite of services meticulously designed to meet the GMP requirements. Their comprehensive approach encompasses:
- GMP audits
- Validation and commissioning
- Consulting on compliance
- Guidance on regulatory submissions
By equipping clients throughout the life science product lifecycle, AVS empowers organizations to adeptly navigate the intricate regulatory landscape while upholding exceptional standards. The recent acquisition of ValidPath significantly bolsters their capabilities in quality engineering, positioning AVS as a trusted partner for regulatory officers in the pharmaceutical sector. This unwavering commitment to excellence is pivotal as the industry encounters heightened regulatory scrutiny and escalating demands for high-quality pharmaceuticals, underscoring the critical importance of for GMP requirements.
How can AVS Life Sciences assist you in achieving compliance excellence today?
Core Principles of Good Manufacturing Practices (GMP)
Good Manufacturing Practices (GMP) are founded on essential principles that guarantee products are consistently produced and controlled to meet high-quality standards. These principles include:
- Quality Management: A robust quality management system (QMS) is crucial for overseeing all production aspects. AVS Life Sciences emphasizes that a well-designed QMS not only meets regulatory requirements but also enhances operational efficiency and product quality. Quality Management System (QMS) software plays a vital role in enhancing GMP adherence by managing documentation, training, processes, procedures, and premises. This integration guarantees adherence to and promotes a culture of continuous improvement.
- Personnel Training: Adequate training for all employees regarding GMP requirements and their specific roles is essential. Regular training programs help ensure that personnel are well-versed in current practices and can effectively contribute to upholding standards.
- Facility and Equipment: Clean and well-maintained facilities and equipment are fundamental to preventing contamination. Regular maintenance and adherence to GMP standards in facility design and layout are necessary to ensure operational integrity.
- Documentation: Comprehensive documentation is a cornerstone of GMP adherence. Maintaining detailed records of all processes not only ensures traceability but also supports accountability during audits and inspections. This includes documenting batch production, laboratory tests, and distribution processes, in line with GXP and FDA regulations.
- Risk Management: Identifying and mitigating risks throughout the manufacturing process is vital for ensuring product safety and efficacy. A proactive strategy for risk management, backed by a well-executed QMS, aids in preventing possible issues before they emerge. Most companies inspected are found to be fully compliant with CGMP regulations, highlighting the importance of maintaining a robust QMS.
To effectively apply GMP principles, regulatory officers should regularly review and update their QMS and training programs to align with the latest GXP and FDA regulations. Continuous improvement and adherence to GMP principles are essential for achieving excellence in pharmaceutical manufacturing. AVS Life Sciences offers specialized solutions in GMP compliance, validation, and engineering, ensuring that organizations can effectively manage the intricacies of GMP requirements and regulatory adherence.
Global Regulatory Frameworks: FDA, EMA, and WHO GMP Standards
Compliance officers must adeptly navigate a variety of global regulatory frameworks, particularly those established by the FDA, EMA, and WHO, each with distinct GMP standards:
- FDA (U.S. Food and Drug Administration): The FDA's current Good Manufacturing Practice (cGMP) regulations outline essential requirements for the manufacturing, processing, and packaging of pharmaceuticals. These regulations highlight the necessity of strong management systems and meticulous documentation to ensure product safety and efficacy. AVS Life Sciences offers comprehensive solutions to help organizations comply with GMP requirements, ensuring that management systems are effectively implemented.
- EMA (European Medicines Agency): The EMA's guidelines emphasize the standard and safety of medicinal products within the European Union. They advocate for a and continuous improvement, ensuring that manufacturers adapt to evolving industry standards and consumer expectations. AVS Life Sciences offers specialized consulting services to help companies align their practices with GMP requirements, promoting a culture of excellence and adherence.
- WHO (World Health Organization): WHO guidelines are intended to improve global health by ensuring that medicines are safe, effective, and of superior standard. The organization concentrates on aligning regulations across various nations, promoting international trade and adherence. AVS Life Sciences plays a crucial role in assisting organizations maneuver through these global benchmarks, ensuring that they fulfill the GMP requirements while upholding high levels of excellence and safety.
Grasping these frameworks is essential for regulatory officers, as they must guarantee that their organizations fulfill the diverse requirements while upholding high standards of excellence and safety.
Common Compliance Challenges in GMP and Strategies to Overcome Them
Compliance officers frequently confront various challenges in adhering to GMP requirements, which can significantly impact product quality and patient safety. Key issues include:
- Documentation Gaps: Inadequate record-keeping poses a prevalent challenge that can lead to compliance failures. A systematic evaluation of current practices against established GMP standards is crucial for identifying gaps. For instance, in a recent case study, AVS Life Sciences assisted a leading biotechnology company in upgrading their manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. Our thorough documentation efforts demonstrated full traceability, which was deemed appropriate by the client’s quality assurance team. The project was completed on schedule and within budget. Organizations should implement robust documentation practices, ensuring that all records are complete, accurate, and readily accessible for audits. Regular reviews and updates of documentation are vital to ensure adherence and traceability throughout the manufacturing process.
- Training Deficiencies: Insufficient training of personnel is a significant contributor to non-compliance. Establishing regular training programs that emphasize GMP requirements and best practices is vital. These programs should include , such as the lessons learned during the aforementioned facility upgrade, to enhance understanding and retention. Involving cross-functional teams in training initiatives can further enhance the culture of adherence within the organization.
- Resource Constraints: Limited resources frequently obstruct adherence initiatives, especially for smaller pharmaceutical firms. Emphasizing adherence in budget allocations and seeking external assistance, such as consulting services from AVS Life Sciences, can help organizations effectively manage regulatory challenges. Investing in technology and tools that streamline documentation and training processes can also alleviate resource burdens.
- Regulatory Changes: The pharmaceutical landscape is continually evolving, with frequent updates to regulations. Establishing a regulatory intelligence system can assist organizations in staying informed about changes and adapting their practices accordingly. Consistently interacting with regulatory agencies and industry organizations can offer insights into forthcoming changes and optimal practices for adherence.
By tackling these challenges proactively, regulatory officers can significantly enhance their organizations' adherence to GMP requirements, ultimately ensuring the safety and quality of pharmaceutical products.
Importance of GMP Training for Pharmaceutical Staff
Training is essential for achieving , equipping pharmaceutical personnel with the knowledge and skills necessary to meet regulatory adherence. Effective GMP training encompasses several key components:
- Understanding GMP Principles: A solid grasp of GMP principles is vital for employees to apply these standards effectively in their daily operations.
- Role-Specific Training: Tailoring training programs to align with specific job functions ensures that staff are well-prepared for their unique responsibilities. Success stories from various organizations illustrate how customized training has led to enhanced compliance and operational efficiency.
- Continuous Education: Regular refresher courses and updates on regulatory changes are crucial for keeping staff informed and compliant. This ongoing education fosters a culture of excellence and adaptability within the organization.
- Assessment and Feedback: Implementing assessments to evaluate the effectiveness of training programs, alongside gathering participant feedback, is critical for continuous improvement. This approach not only elevates training standards but also aligns with the evolving needs of the industry.
Integrating these components into GMP training programs can significantly enhance adherence results and cultivate a proactive attitude toward the management of GMP requirements.
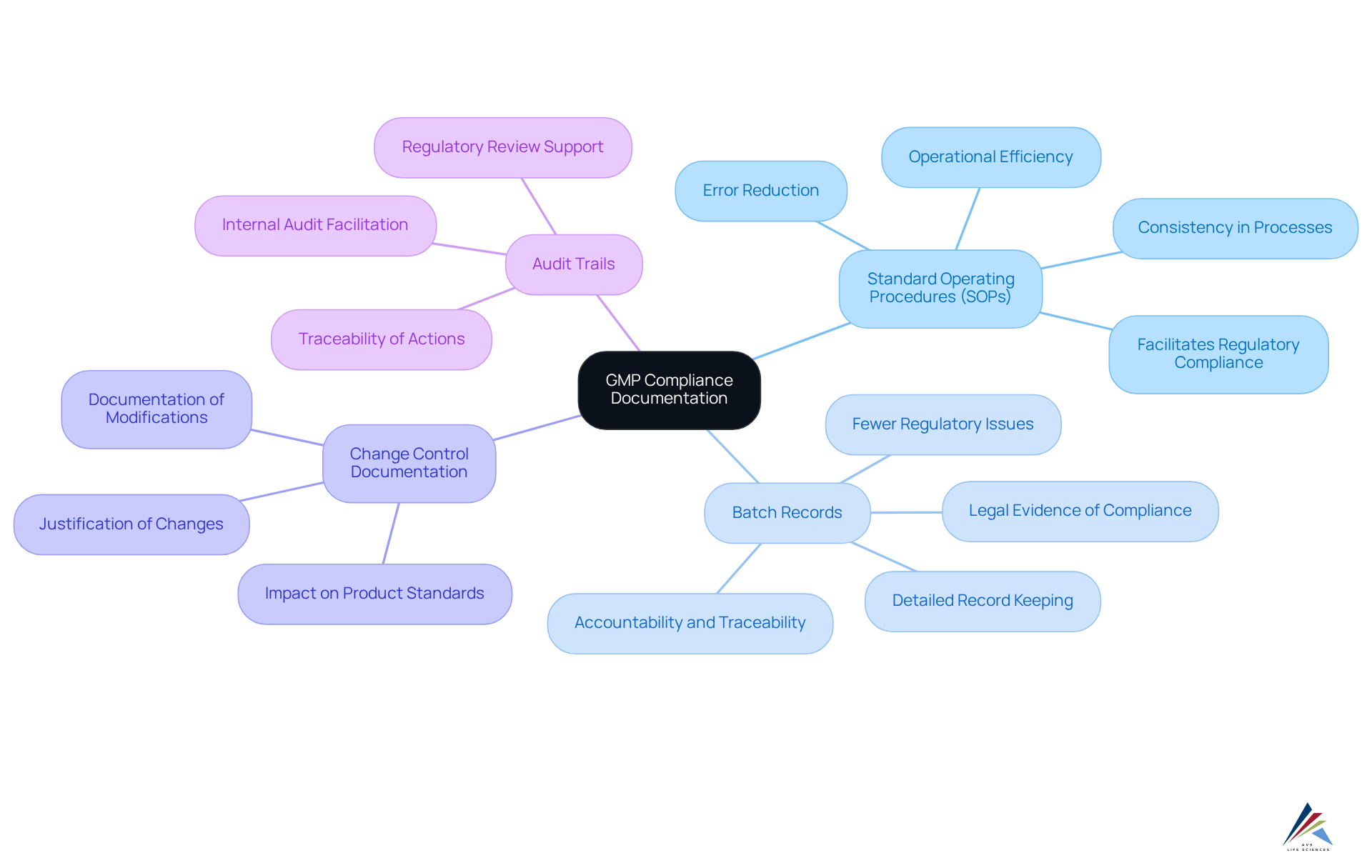
Documentation and Record-Keeping Requirements for GMP Compliance
Documentation and record-keeping are pivotal foundations of GMP adherence, ensuring that pharmaceutical manufacturing processes not only meet GMP requirements but also uphold product quality. Key requirements include:
- Standard Operating Procedures (SOPs): Establishing clearly defined SOPs for all processes is imperative. These documents not only ensure consistency but also facilitate compliance with regulatory expectations. A well-implemented SOP framework can significantly reduce errors and enhance operational efficiency. AVS Life Sciences offers tailored SOP development services to assist organizations in aligning with GMP standards effectively.
- Batch Records: Maintaining detailed records for each batch produced is essential. These records should encompass raw material usage, processing steps, and control results. Accurate batch records serve as legal evidence that GMP protocols were followed, supporting accountability and traceability throughout the production process. AVS Life Sciences emphasizes the significance of robust batch record practices, which can lead to fewer regulatory issues and enhanced operational efficiency.
- Change Control Documentation: Any modifications to processes or equipment must be meticulously documented and justified. This practice is crucial for upholding regulations and ensuring that changes do not adversely affect product standards or safety. AVS Life Sciences provides consulting services to assist companies in establishing effective change control processes.
- Audit Trails: Comprehensive audit trails are necessary to ensure traceability and accountability in all operations. These records should detail every action taken during the manufacturing process, providing a clear path for regulatory review and internal audits. AVS Life Sciences can aid organizations in implementing systems that ensure thorough audit trails are maintained.
The implementation of SOPs and not only fosters a culture of quality and continuous improvement within organizations but also aligns with GMP requirements. Statistics indicate that companies with strong SOPs and batch record practices encounter fewer regulatory issues and achieve greater operational efficiency, underscoring the importance of these factors in the pharmaceutical sector. Furthermore, the PICS GMP Guide section 4.15 mandates that processing instructions include specific details, reinforcing the regulatory framework that supports thorough documentation.
Conducting Effective GMP Audits: Best Practices
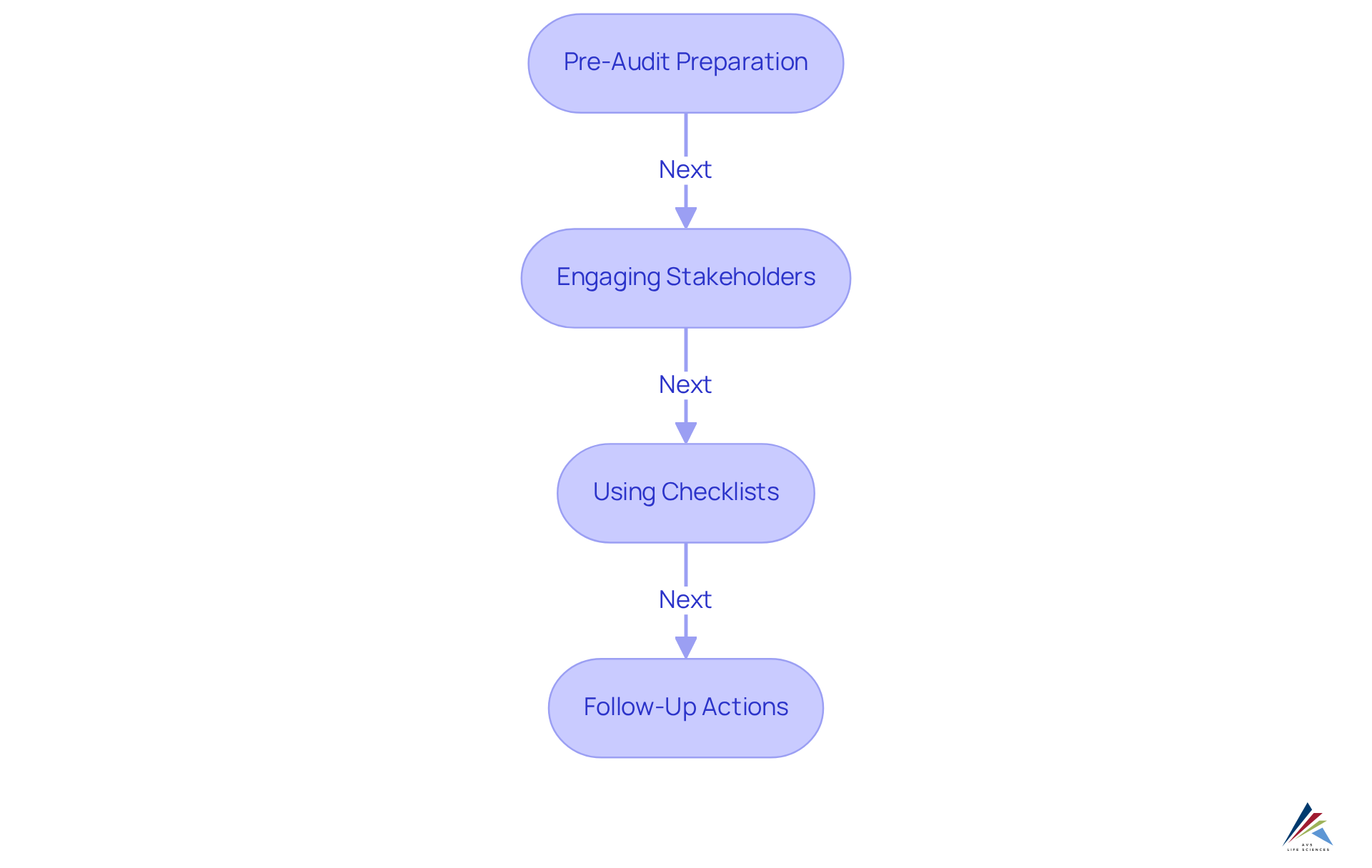
Conducting effective audits in accordance with GMP requirements requires meticulous planning and execution. Key best practices include:
- Pre-Audit Preparation: Thoroughly review previous audit findings and relevant documentation to pinpoint potential areas of concern. This proactive approach significantly enhances audit readiness and mitigates risks associated with non-compliance with GMP requirements.
- Engaging Stakeholders: Actively involve key personnel in the audit process to foster transparency and cooperation. Engaging stakeholders ensures that everyone understands their roles and responsibilities, which is crucial for a smooth audit experience.
- Using Checklists: Create comprehensive audit checklists to guarantee that all critical areas are addressed during the audit. Checklists serve as valuable tools to streamline the audit process and ensure no essential aspect is overlooked.
- Follow-Up Actions: Post-audit, it is vital to implement and document corrective actions to resolve any identified issues. This step not only tackles urgent issues but also aids in the ongoing enhancement of regulatory practices.
Recent statistics highlight the growing scrutiny in the pharmaceutical sector, with the number of Form 483s issued to drug establishments increasing by approximately 116% from FY2021 to FY2022. This underscores the necessity for that comply with GMP requirements. Furthermore, industry experts emphasize that audits are indispensable for driving continual enhancements within pharmaceutical entities, providing insights that refine procedures and frameworks. By emphasizing efficient audit preparation, businesses can maintain standards and successfully manage the intricacies of regulatory adherence.
Implementing Quality Control Measures in GMP Compliance
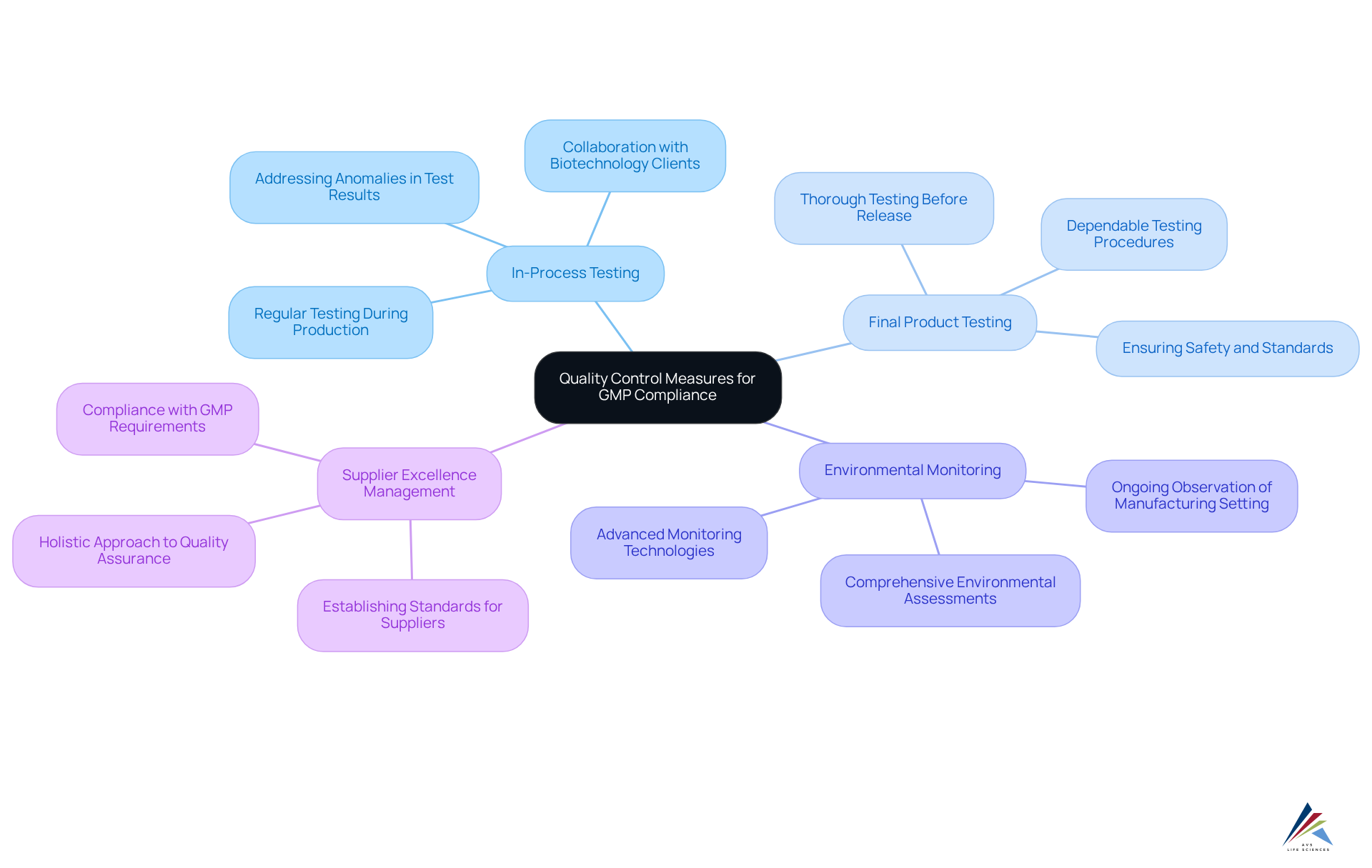
Establishing strong control procedures is crucial for guaranteeing adherence to GMP requirements. Key strategies include:
- In-Process Testing: Regular testing during production is essential for identifying and addressing potential issues early. A collaboration between AVS Life Sciences and a leading biotechnology client underscored the importance of thorough in-process testing. Anomalies in test results were identified during the upgrade of their GMP facility, particularly due to overlooked barcode scanner installations.
- Final Product Testing: All completed items must undergo thorough testing to confirm their standards and safety before release. AVS Life Sciences assisted the client in creating dependable testing procedures, ensuring that medications produced with lentivirus vector material met strict criteria. This approach preserves consumer trust and ensures regulatory adherence.
- Environmental Monitoring: Ongoing observation of the manufacturing setting is essential to avoid contamination and guarantee adherence to cleanliness criteria. The upgrade process included comprehensive environmental assessments and the implementation of advanced monitoring technologies. This enhancement improved and led to quicker responses to potential contamination risks.
Supplier Excellence Management is critical for establishing stringent standards for suppliers to ensure that raw materials meet GMP requirements. AVS Life Sciences emphasizes the importance of supplier quality management as part of a holistic approach to quality assurance. This ensures that all components of the manufacturing process comply with GMP requirements.
Personnel Qualifications and Training in GMP Compliance
Personnel qualifications and training are paramount for achieving compliance with GMP requirements. To address compliance challenges effectively, several key considerations must be prioritized:
- Education and Experience: All personnel must possess the requisite education and experience to fulfill their roles effectively. This foundational knowledge ensures that employees grasp the complexities of the GMP requirements and can apply them proficiently in practice.
- Role-Specific Training: Tailoring training to the specific responsibilities of each employee significantly enhances their understanding of GMP requirements. For instance, training programs focused on the unique needs of aseptic operators have demonstrated a ≥90% quiz pass rate, underscoring the effectiveness of targeted education.
- Continuous Professional Development: Ongoing education is essential for keeping staff updated on the latest GMP requirements and practices. Regular refresher courses are necessary, as they reinforce critical concepts and adapt to evolving regulations, fostering a culture of continuous improvement.
- Performance Evaluations: Conducting regular assessments of employee performance is crucial for ensuring adherence to GMP standards. These evaluations not only identify areas for improvement but also promote accountability among staff, which is essential for meeting GMP requirements and maintaining high-quality manufacturing processes.
Integrating these elements into training programs not only enhances adherence but also mitigates risks linked to non-compliance, such as steep fines and penalties. The case of ACRL illustrates the critical importance of prioritizing GMP from the outset to avoid jeopardizing business operations, emphasizing the need for effective training and documentation practices.

Recent Trends and Developments in Good Manufacturing Practices
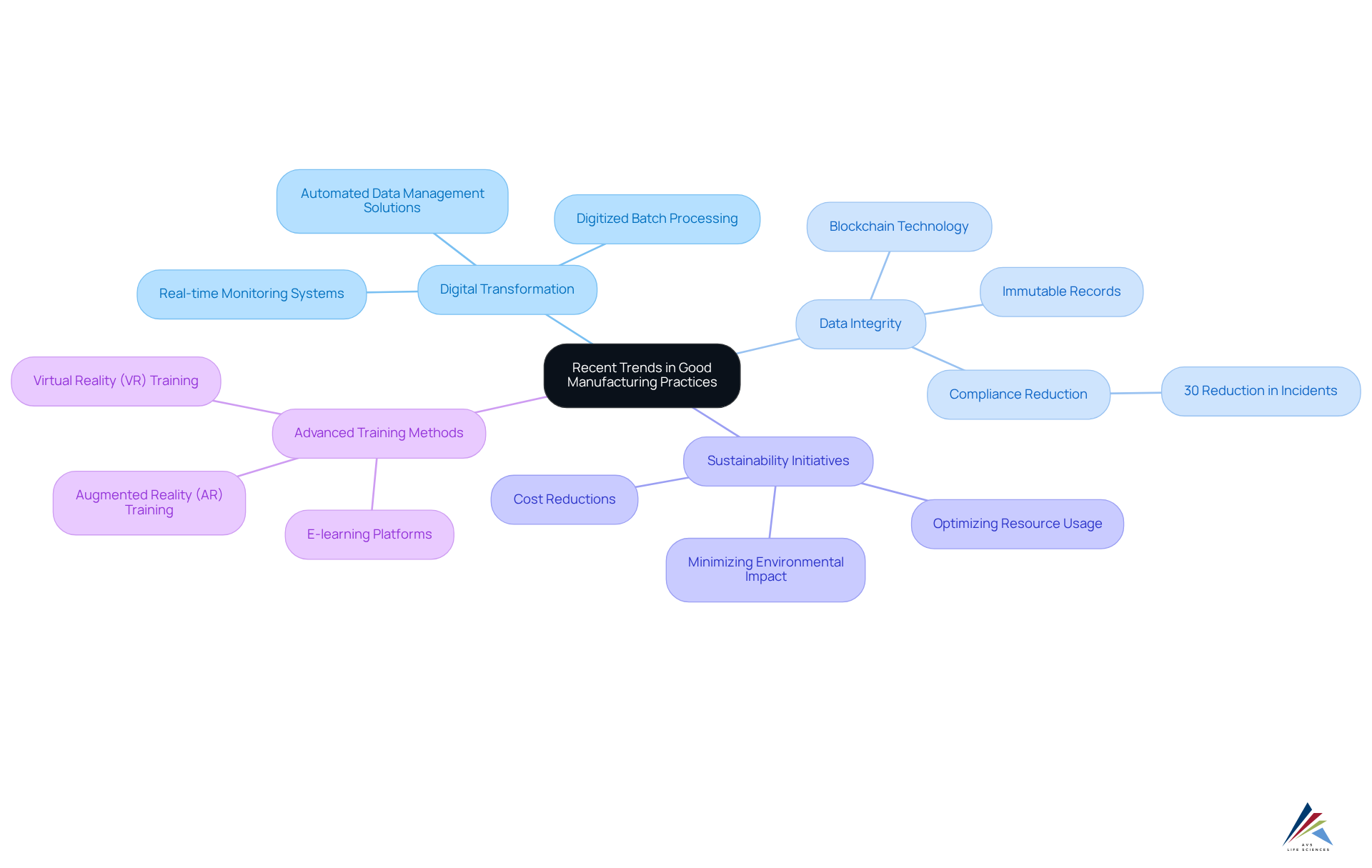
Recent trends and developments in Good Manufacturing Practices (GMP) reflect significant advancements in the pharmaceutical industry:
- Digital Transformation: The integration of digital tools and technologies is revolutionizing GMP compliance. Real-time monitoring systems and automated data management solutions enhance operational efficiency and ensure adherence to regulatory standards. For instance, AVS Life Sciences successfully assisted a leading biotechnology company in upgrading their manufacturing space to a Level 2 GMP facility, implementing digitized batch processing solutions that improved data collection and operational transparency. This seamless integration of digital technologies not only streamlines processes but also reduces the risk of human error, ultimately leading to improved product quality.
- Focus on Data Integrity: Regulatory bodies are increasingly prioritizing data integrity, compelling organizations to adopt stringent data management practices. Initiatives such as blockchain technology are being utilized to create immutable records, enhancing traceability and adherence throughout the supply chain. This shift not only but also mitigates the risk of counterfeit products. According to recent studies, companies that implement robust data integrity measures can reduce compliance-related incidents by up to 30%. AVS Life Sciences' expertise in incorporating advanced data management systems has demonstrated its importance in assisting clients' adherence initiatives.
- Sustainability Initiatives: There is a notable trend towards sustainable manufacturing practices. Businesses, such as AVS Life Sciences, are actively striving to minimize their environmental impact while ensuring compliance with GMP requirements. This includes optimizing resource usage and minimizing waste, which aligns with both regulatory expectations and corporate social responsibility goals. Adopting sustainable methods can also result in cost reductions, further encouraging adherence.
- Advanced Training Methods: The rise of e-learning and virtual training platforms is transforming GMP training for pharmaceutical personnel. These innovative training approaches enhance accessibility and effectiveness, allowing staff to remain informed about regulatory requirements and best practices. Enhanced training through augmented reality (AR) and virtual reality (VR) technologies further supports skill development, leading to better adherence to GMP requirements and reduced human error. Compliance officers are encouraged to leverage these technologies to enhance their training programs and ensure their teams are well-prepared for evolving regulatory landscapes.
In summary, by adopting these trends, compliance officers can greatly improve their organizations' efforts to meet GMP requirements, ensuring both operational efficiency and regulatory compliance. AVS Life Sciences plays a crucial role in guiding organizations through these advancements, providing expertise in quality management and regulatory compliance.
Conclusion
Good Manufacturing Practices (GMP) are vital for ensuring the safety and quality of pharmaceuticals. Compliance officers are crucial in navigating the complexities of GMP requirements, which encompass a variety of principles and practices designed to uphold high standards throughout the manufacturing process. By understanding and implementing these core principles, organizations can significantly bolster their compliance efforts and ultimately safeguard patient safety.
The article delineates several key aspects of GMP, highlighting the importance of:
- Quality management systems
- Personnel training
- Comprehensive documentation
- Effective risk management
- Adherence to global regulatory frameworks established by the FDA, EMA, and WHO
Furthermore, it tackles common compliance challenges and provides strategies to overcome them, underscoring the necessity of continuous education and robust audit practices. The integration of digital tools and a focus on data integrity further illuminate the evolving landscape of GMP compliance.
As the pharmaceutical industry faces heightened scrutiny and regulatory demands, the importance of adhering to GMP standards cannot be overstated. Compliance officers are urged to leverage specialized consulting services, such as those offered by AVS Life Sciences, to enhance their organizations' compliance frameworks. By prioritizing GMP adherence, organizations not only ensure the quality of their products but also contribute to the overall safety and trustworthiness of the pharmaceutical sector.
Frequently Asked Questions
What services does AVS Life Sciences provide to meet GMP requirements?
AVS Life Sciences offers a suite of services including GMP audits, validation and commissioning, consulting on compliance, and guidance on regulatory submissions.
How does AVS Life Sciences support organizations in the pharmaceutical sector?
AVS Life Sciences empowers organizations by equipping them throughout the life science product lifecycle, helping them navigate regulatory landscapes and maintain exceptional standards, especially in light of increased scrutiny and demand for high-quality pharmaceuticals.
What are the core principles of Good Manufacturing Practices (GMP)?
The core principles of GMP include Quality Management, Personnel Training, Facility and Equipment maintenance, Documentation, and Risk Management, all aimed at ensuring products are consistently produced and controlled to meet high-quality standards.
Why is a Quality Management System (QMS) important in GMP?
A QMS is crucial for overseeing all production aspects, enhancing operational efficiency, ensuring compliance with regulatory requirements, and promoting continuous improvement in product quality.
What role does documentation play in GMP adherence?
Comprehensive documentation ensures traceability and accountability during audits and inspections, including detailed records of batch production, laboratory tests, and distribution processes.
How do global regulatory frameworks like FDA, EMA, and WHO influence GMP compliance?
These frameworks establish distinct GMP standards that organizations must navigate to ensure product safety and efficacy, with AVS Life Sciences providing solutions to help companies comply with these regulations.
What are the FDA's requirements regarding GMP?
The FDA's cGMP regulations highlight the need for strong management systems and meticulous documentation in the manufacturing, processing, and packaging of pharmaceuticals.
How does AVS Life Sciences assist with compliance to EMA guidelines?
AVS Life Sciences offers specialized consulting services to help companies align their practices with EMA GMP requirements, promoting a culture of excellence and continuous improvement.
What is the focus of WHO guidelines in relation to GMP?
WHO guidelines aim to ensure that medicines are safe, effective, and of high standard, promoting international trade and adherence to regulations across nations. AVS Life Sciences helps organizations meet these global benchmarks.
