10 Essential GMP Regulations for Pharmaceutical Compliance Officers

Overview
The article emphasizes the critical GMP regulations that pharmaceutical compliance officers must comprehend to ensure strict adherence to industry standards. Understanding these regulations is vital for maintaining product safety and quality, as they encompass extensive guidelines, documentation practices, and training that collectively enhance operational efficiency and foster consumer trust in pharmaceutical products. By grasping these essential regulations, compliance officers can navigate the complexities of the industry, ensuring that their organizations not only meet but exceed compliance expectations.
Introduction
Navigating the intricate landscape of Good Manufacturing Practices (GMP) is essential for pharmaceutical compliance officers who are tasked with ensuring product safety and regulatory adherence. This article explores ten critical GMP regulations that not only safeguard public health but also enhance operational efficiency and consumer trust. As the pharmaceutical industry confronts evolving standards and increased scrutiny, compliance officers must effectively implement these regulations to mitigate risks and foster a culture of excellence.
AVS Life Sciences: Comprehensive GMP Compliance Solutions for Pharmaceutical Operations
AVS Life Sciences offers a comprehensive suite of services designed to ensure strict adherence to across pharmaceutical operations. Their meticulous approach includes:
- Contract test laboratories
- Various manufacturing, storage, and distribution sites
This ensures that every aspect of the production process meets the highest standards of quality and compliance.
to navigate the complexities of GMP regulations with ease, supported by a team of over 300 seasoned professionals. They provide , , and engineering support, all customized to meet the unique needs of clients in the life sciences sector. This dedication not only enhances but also fosters trust within the regulatory environment.
Recognizing the about , AVS Life Sciences encourages regulatory officers to subscribe to the ECA's complimentary GMP newsletters. These newsletters cover a wide range of relevant topics, ensuring that professionals remain at the forefront of compliance.
Additionally, the recent acquisition of ValidPath significantly bolsters AVS's capabilities in , further solidifying its expertise in solutions for the global life sciences industry, particularly in GMP regulations and oversight. Engage with AVS Life Sciences to elevate your compliance strategy and with the highest industry standards.
Current Good Manufacturing Practices (CGMP): Ensuring Product Safety and Quality
(C) are essential regulations that ensure pharmaceutical products are consistently manufactured and regulated according to established standards. , along with GXP and FDA regulations, is vital for maintaining data integrity and implementing effective . These practices are instrumental in preventing contamination, mix-ups, and errors, thereby . To navigate these complexities, pharmaceutical oversight officials must possess comprehensive knowledge of and related frameworks to guarantee that all manufacturing processes adhere to stringent assurance standards and legal requirements.
AVS Life Sciences stands at the forefront of compliance, validation, and engineering in accordance with GMP regulations. Our designed to elevate management standards and ensure . By engaging with AVS Life Sciences, organizations can enhance their operational integrity and align with the highest industry standards, ultimately that protects public health.
Regulatory Framework: Understanding GMP Guidelines and Compliance Requirements
The framework for is shaped by guidelines from leading authorities such as the FDA and EMA, which establish critical standards for , control of standards, and documentation. must possess a thorough understanding of [GMP regulations](https://avslifesciences.com/industry-news/advancements-in-medical-research) to ensure their organizations meet the necessary benchmarks, thereby mitigating risks associated with non-compliance, including penalties and product recalls.
For example, the FDA enforces (cGMP) guidelines that stress systematic procedures to ensure product quality, while the EMA conducts rigorous audits to verify adherence to GMP regulations across the EU. Recent developments, such as the ICH's draft M4Q(R2) guideline, aim to modernize the (CTD) Quality Modules, aligning submissions with Quality by Design (QbD) principles and digital data expectations.
This evolution in oversight expectations highlights the necessity for compliance officers to remain informed and proactive in their approach to . Organizations that neglect GMP regulations face significant repercussions, including substantial fines and damage to their reputation. Conversely, those that effectively implement GMP regulations, as exemplified by , not only enhance product safety and efficacy but also cultivate trust with regulatory bodies and consumers alike.
A transformative case study involving [AVS Life Sciences](http://avslifesciences.com/blog-post/7-ways-how-avs-supports-brands-with-compliant-packaging-and-label-review) illustrates this point: the company successfully assisted a leading biotechnology firm in upgrading their manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility, thereby enabling the successful manufacturing of medication with lentivirus vector material.
According to the GMP Insiders Expert Team, the of pharmaceutical and medical device products are guaranteed by GMP regulations. To ensure compliance, organizations should routinely review their processes and engage in continuous training for their teams.
Quality Control Measures: Key Components of GMP Regulations
Essential elements of encompass stringent control measures, including batch testing, stability studies, and . Compliance officers play a pivotal role in ensuring that these measures are effectively executed in , thus throughout the manufacturing process.
Regular ; it aids in identifying deviations and potential problems early, allowing for prompt corrective measures that ensure compliance with [gmp regulations](https://avslifesciences.com/case-studies/cell-gene-therapy). Stability studies further contribute by evaluating how product standards are maintained over time under varying environmental conditions, ensuring that pharmaceutical products comply with gmp regulations to remain safe and effective throughout their shelf life.
Adhering to GMP regulations, GXP, and FDA regulations, along with robust documentation practices, is vital for maintaining compliance and avoiding .
A notable case study involves in upgrading their manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility for lentivirus production. This upgrade was finalized on time and within budget, showcasing the effectiveness of .
Companies that prioritize these GMP regulations not only comply with regulatory requirements but also enhance their reputation by consistently delivering high-quality products. To guarantee ongoing adherence, it is essential for oversight officers to routinely assess and revise , promoting a culture of continuous enhancement within their organizations.
Documentation and Record-Keeping: Critical Aspects of GMP Compliance
are critical components of , as they serve as evidence of . Compliance officers must ensure that every aspect of manufacturing processes, control tests, and deviations is documented with precision.
As a leading provider of management and oversight solutions in the life sciences sector, designed to enhance in accordance with GMP regulations. This not only supports adherence during inspections but also serves as a valuable asset for continuous improvement initiatives.
By maintaining accurate records, organizations can demonstrate their commitment to , ultimately fostering a culture of excellence within the pharmaceutical manufacturing environment.
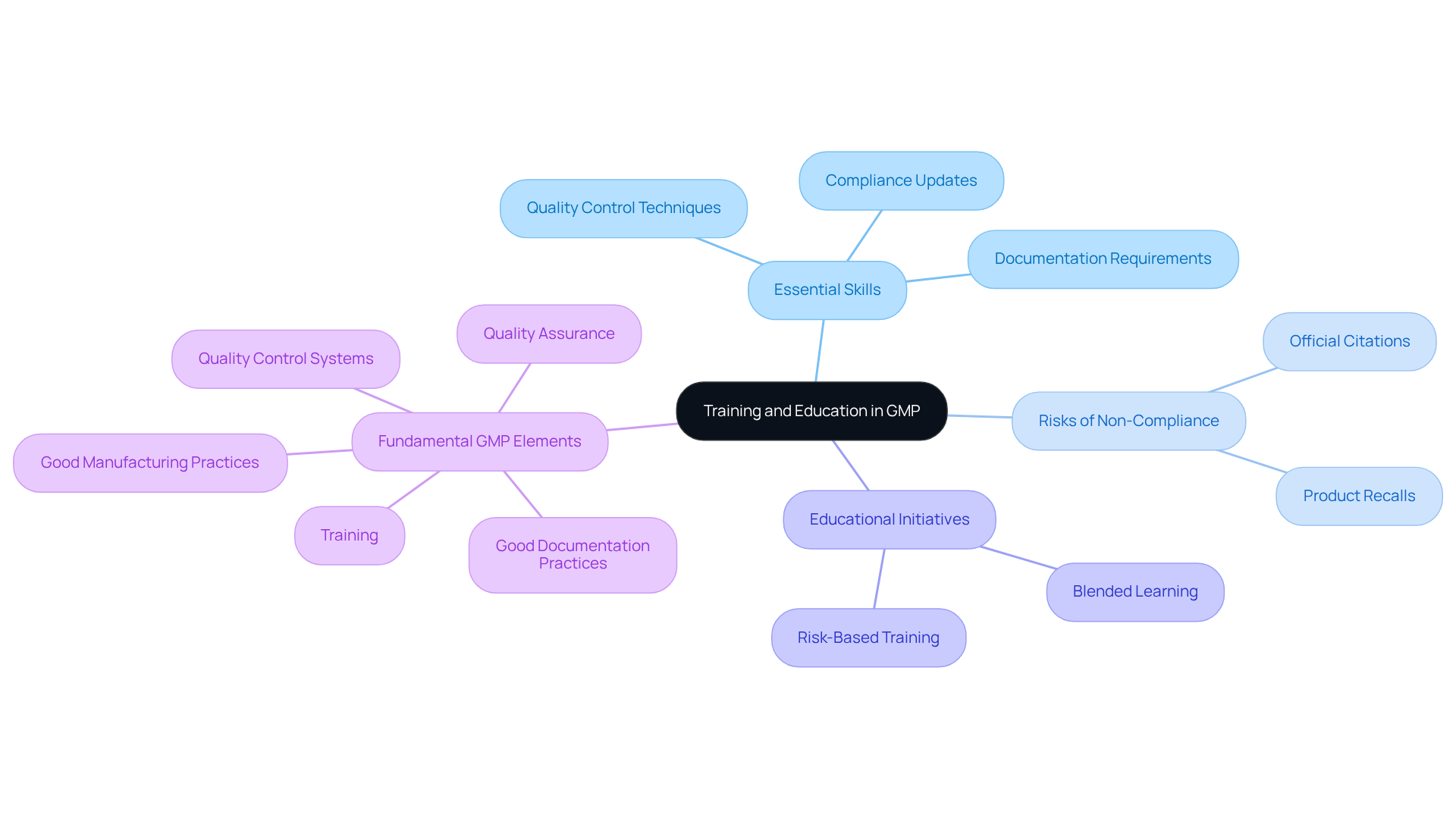
Training and Education: Empowering Compliance Officers in GMP Practices
Training and education are essential for equipping oversight officers with the necessary skills to implement (GMP) effectively. Regular training sessions must encompass the latest compliance updates, , and documentation requirements. This ensures that personnel stay informed about evolving standards, including GXP and FDA regulations. A culture of continuous learning not only enhances the capabilities of regulatory teams but also fosters an environment where compliance with becomes second nature.
Organizations that prioritize continuous learning can significantly reduce risks associated with non-compliance, such as product recalls and official citations. Successful educational initiatives, including blended learning approaches and risk-based training programs, have demonstrated effectiveness in reinforcing GMP regulations and improving overall operational efficiency.
As the landscape of pharmaceutical production evolves in 2025, the importance of cannot be overstated; they are critical for maintaining high-quality standards and ensuring compliance with GMP regulations. Furthermore, the necessity of , particularly for personnel in sterile manufacturing environments, who must engage in regular training concerning PIC/S Annex 1 standards.
The five fundamental elements of GMP regulations—Quality Assurance, Good Manufacturing Practices, Good Documentation Practices, Quality Control Systems, and Training—should be integral to any training program, ensuring that oversight officers are well-equipped to meet legal expectations.
AVS Life Sciences provides extensive biopharmaceutical and life sciences consulting services, focusing on management standards, regulatory compliance, and engineering solutions, all of which are essential for the efficient execution of GMP regulations.
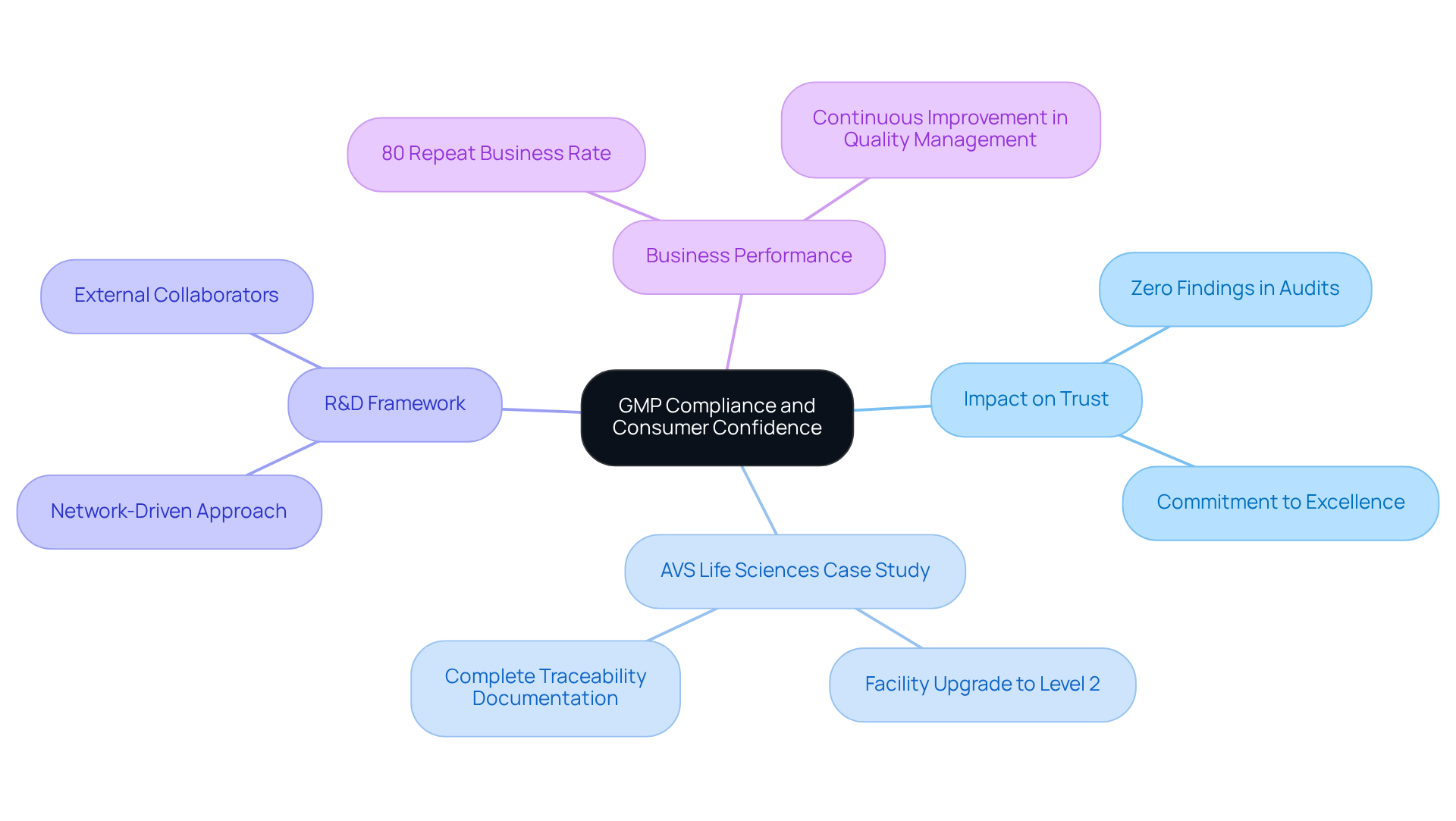
Consumer Confidence: The Impact of GMP Compliance on Market Trust
is essential for cultivating consumer confidence in pharmaceutical products. Organizations that adhere to not only demonstrate their and safety but also significantly enhance their market reputation. Compliance officers must recognize that their efforts directly influence consumer trust, necessitating a steadfast commitment to uphold the . For instance, companies that consistently achieve exemplify reliability, which in turn fosters trust among consumers and stakeholders alike.
A transformative example of this is of a biotechnology GMP facility, where they assisted a leading San Francisco-based biotechnology company in transitioning from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. This project was completed on time and within budget, underscoring AVS's dedication to excellence in management and regulatory adherence. Significantly, our documentation efforts to illustrate complete traceability were deemed suitable by the client’s assurance team.
Moreover, the shift to a network-driven R&D framework in the pharmaceutical sector underscores the importance of maintaining stringent control measures, as companies increasingly rely on external collaborators to uphold GMP regulations. This interconnected approach to managing standards enhances information sharing and improves overall outcomes, reinforcing the notion that excellence is a collective responsibility throughout the organization.
Ultimately, the commitment to GMP regulations not only safeguards patient health but also reinforces a company's standing in a competitive market, as evidenced by AVS Life Sciences' impressive . Additionally, crucial lessons learned from anomalies in test results have led to evaluations of business processes, emphasizing the importance of continuous improvement in .
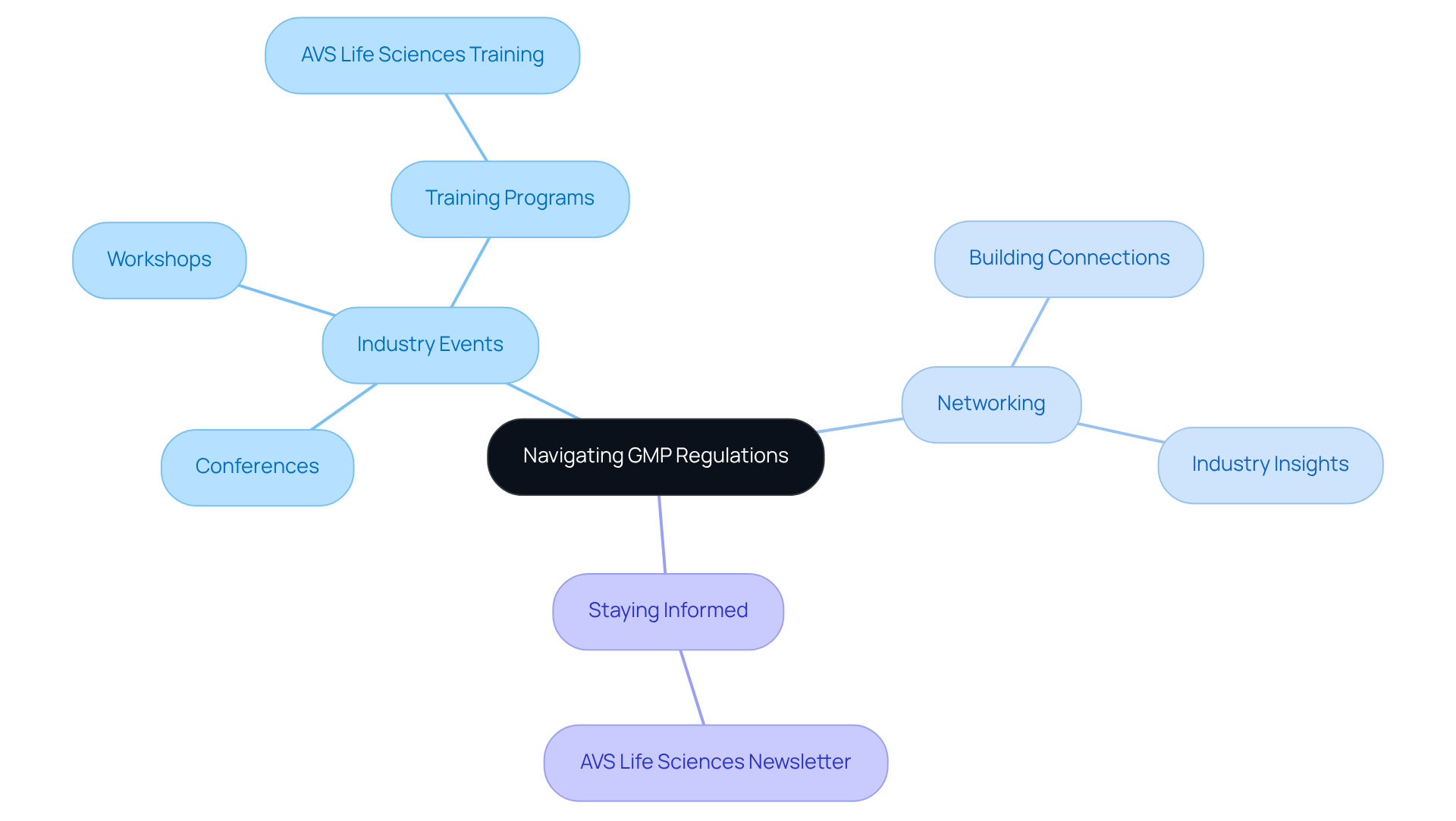
Navigating Challenges: Keeping Up with Evolving GMP Regulations
Navigating the complexities of evolving GMP regulations necessitates that oversight officers remain vigilant and well-informed about industry trends and updates. It is essential for professionals to participate in , workshops, and , such as those offered by AVS Life Sciences, to stay abreast of the . These events not only deliver vital updates but also create that can provide invaluable insights and support in adapting to new GMP regulations.
By cultivating a robust network of industry connections, compliance officers can enhance their ability to respond effectively to , ensuring their organizations remain compliant and competitive in a rapidly changing landscape. To stay informed about , subscribe to AVS Life Sciences' newsletter, which connects you with the and offers insights into the latest regulatory developments.
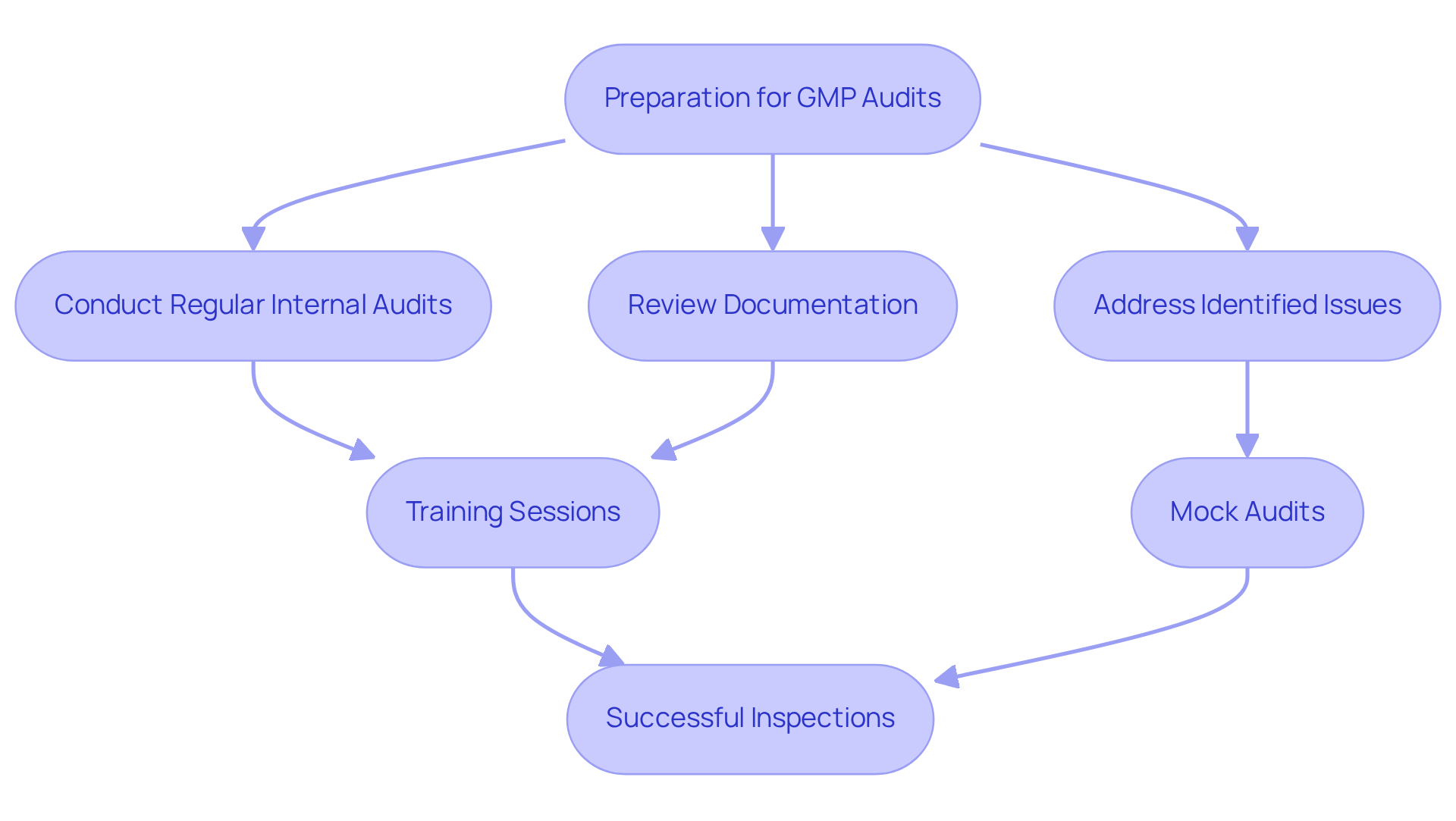
Audits and Inspections: Ensuring Adherence to GMP Standards
Audits and inspections are pivotal in ensuring strict adherence to . For regulatory officers, meticulous preparation is paramount for . This preparation includes:
- Conducting regular
- Thoroughly reviewing documentation
- Proactively addressing any identified issues
Organizations that prioritize these practices not only demonstrate their commitment to [GMP regulations](http://avslifesciences.com/blog-post/10-leading-biotech-consulting-firms-for-compliance-solutions) but also significantly mitigate the risk of non-conformities during external inspections.
Recent trends indicate that are essential for compliance, with recommended to keep staff well-informed and prepared. Furthermore, maintaining and ensuring clarity in employee responsibilities can effectively reduce regulatory issues stemming from inadequate training.
AVS Life Sciences provides robust support to ensure , reinforcing its expertise in this critical area. Effective strategies for preparing for GMP inspections include:
- Establishing clear procedures for reporting adherence issues, which fosters transparency and accountability under GMP regulations
- Regular equipment calibration
- [Internal audits](https://linkedin.com/pulse/best-practices-effective-internal-auditing-facilities-troy-fugate-vpf7e) as vital components of a solid adherence strategy
By implementing these strategies, organizations can enhance their audit readiness and facilitate a seamless inspection process. For tailored consulting solutions, regulatory officers are encouraged to engage with AVS Life Sciences.
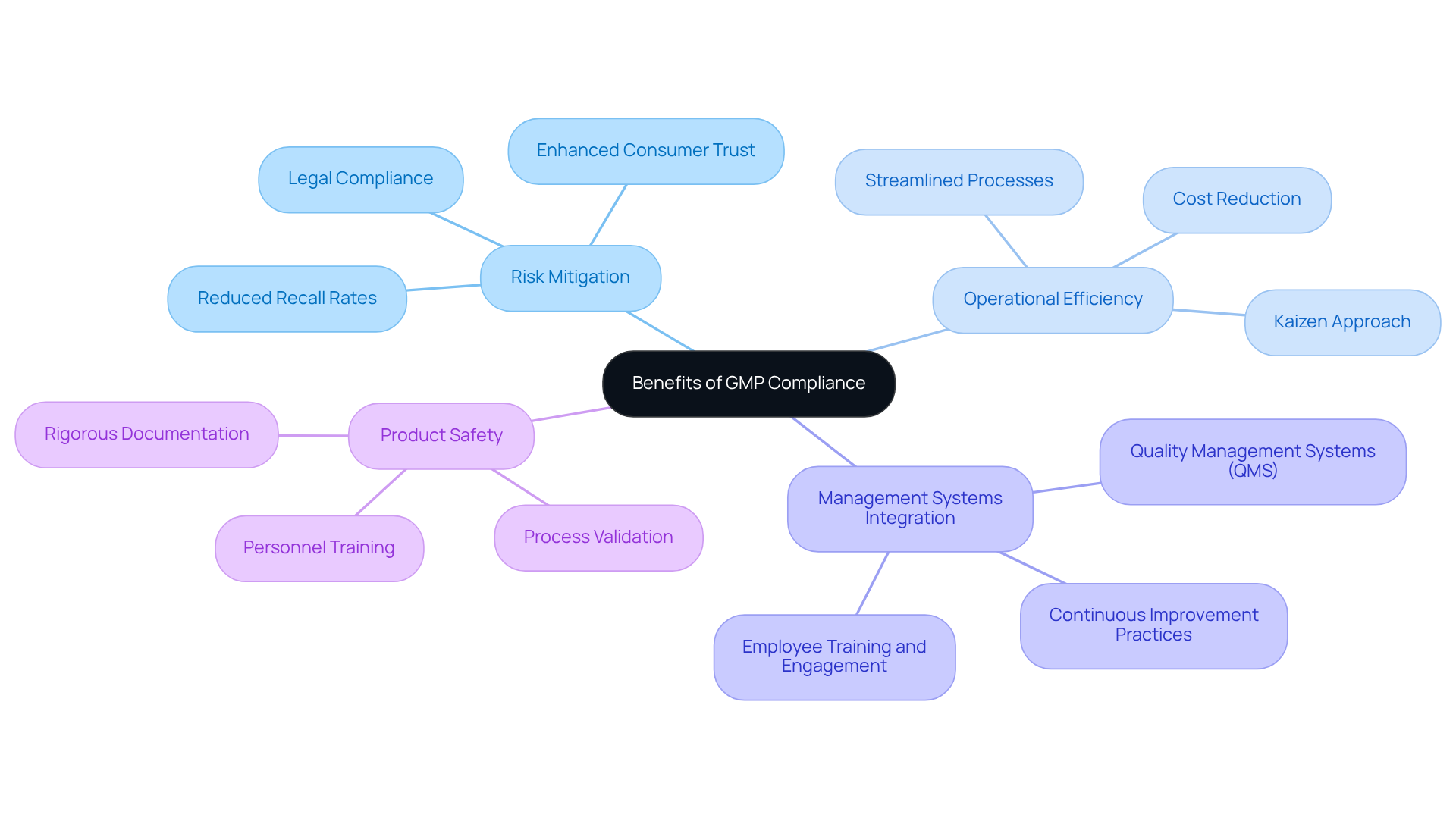
Benefits of GMP Compliance: Enhancing Quality and Safety in Pharmaceuticals
provide substantial benefits that extend beyond mere regulatory adherence, fundamentally elevating the standard and safety of pharmaceutical products. By embracing GMP practices, organizations can significantly mitigate the risk of product recalls, which can result in costly legal consequences and reputational damage. For instance, in assisting a leading biotechnology company to upgrade its manufacturing facility from a Biosafety Level 1 GMP environment to a Level 2 GMP facility. This upgrade not only demonstrated stringent control measures but also resulted in a marked , thereby bolstering consumer trust and confidence in their products.
Moreover, GMP regulations enhance by and minimizing waste. This is particularly evident in organizations that adopt continuous improvement methodologies, such as the Kaizen approach, which focuses on small, incremental changes that collectively boost productivity. Consequently, AVS Life Sciences' comprehensive support during this transition allowed the client to focus on medicine development while ensuring that their control laboratory meticulously assessed and refined their processes. This led to improved resource utilization and reduced operational costs, ultimately enhancing profitability.
Current trends reveal a growing emphasis on integrating management systems (QMS) that align with GMP regulations, fostering a culture of excellence throughout the organization. This holistic approach not only improves compliance but also encourages innovation and responsiveness to market demands.
The impact of GMP regulations on product safety is profound, as these regulations establish rigorous protocols for documentation, , and personnel training. Routine evaluations and oversight under GMP, as illustrated in AVS Life Sciences' case study, empower organizations to identify potential issues early, preventing defective products from reaching the market. This proactive stance is essential for maintaining high standards of patient safety and ensuring that pharmaceutical products consistently meet performance criteria.
In conclusion, the implementation of GMP regulations is vital for regulatory officers aiming to , operational efficiency, and consumer trust, while simultaneously . Furthermore, it is crucial to acknowledge that is a legal obligation enforced by regulatory agencies worldwide, highlighting the necessity of adherence to these standards beyond mere operational advantages.
Conclusion
GMP regulations are essential for ensuring the safety, quality, and efficacy of pharmaceutical products. Compliance officers, by adhering to these critical guidelines, play a vital role in safeguarding public health and fostering trust within the pharmaceutical industry. Commitment to Good Manufacturing Practices not only aligns organizations with regulatory requirements but also enhances their operational integrity and market reputation.
Key aspects of GMP compliance have been underscored, including:
- Rigorous documentation
- Quality control measures
- Continuous training
- Proactive engagement with evolving regulations
Case studies, such as those presented by AVS Life Sciences, illustrate how organizations successfully navigate these complexities, ultimately leading to improved product safety and operational efficiency.
As the landscape of pharmaceutical manufacturing evolves, the emphasis on GMP compliance remains paramount. Organizations are encouraged to invest in comprehensive training programs and adopt a culture of continuous improvement, ensuring they not only meet but exceed regulatory expectations. By doing so, they position themselves as leaders in the industry, committed to delivering high-quality products that consumers can trust.
Frequently Asked Questions
What services does AVS Life Sciences provide for GMP compliance?
AVS Life Sciences offers a comprehensive suite of services including GMP audits for Active Pharmaceutical Ingredient (API) and drug product Contract Manufacturing Organizations (CMOs), contract test laboratories, and support for various manufacturing, storage, and distribution sites.
How does AVS Life Sciences support organizations in navigating GMP regulations?
AVS Life Sciences provides tailored validation, quality assurance consulting, and engineering support to help organizations meet their unique needs in the life sciences sector, enhancing operational efficiency and fostering trust within the regulatory environment.
Why is it important for regulatory officers to stay informed about GMP regulations?
Staying informed about evolving GMP regulations is crucial for regulatory officers to ensure compliance and maintain product safety and quality. AVS Life Sciences encourages subscribing to the ECA's complimentary GMP newsletters for updates on relevant topics.
What role does ValidPath play in AVS Life Sciences' capabilities?
The acquisition of ValidPath enhances AVS Life Sciences' engineering excellence and solidifies its expertise in providing solutions for GMP regulations and oversight within the global life sciences industry.
What are Current Good Manufacturing Practices (CGMP)?
CGMP regulations are essential standards that ensure pharmaceutical products are consistently manufactured and regulated according to established practices, helping to prevent contamination, mix-ups, and errors to safeguard public health.
How do GMP regulations impact pharmaceutical oversight officials?
Pharmaceutical oversight officials must have a comprehensive understanding of GMP regulations to ensure all manufacturing processes adhere to stringent assurance standards and legal requirements, mitigating risks associated with non-compliance.
What are some consequences of neglecting GMP regulations?
Organizations that neglect GMP regulations face significant repercussions, including penalties, product recalls, and damage to their reputation.
Can you provide an example of AVS Life Sciences' impact on GMP compliance?
AVS Life Sciences successfully assisted a leading biotechnology firm in upgrading their manufacturing facility from a Biosafety Level 1 GMP facility to a Level 2 GMP facility, enabling the successful manufacturing of medication with lentivirus vector material.
What should organizations do to ensure ongoing GMP compliance?
Organizations should routinely review their processes and engage in continuous training for their teams to maintain compliance with GMP regulations.
