10 Essential Features of CTMS Systems for Compliance Success
Overview
This article identifies the essential features of Clinical Trial Management Systems (CTMS) that are pivotal for achieving compliance success. It is crucial to recognize the compliance challenges faced in clinical trials. Effective CTMS systems stand out by integrating quality management practices, fostering stakeholder coordination, and automating processes. These elements collectively enhance regulatory adherence and operational efficiency. By implementing such systems, organizations can significantly improve their compliance outcomes. Engaging with AVS Life Sciences can provide the necessary support to navigate these complexities and ensure successful clinical trial management.
Introduction
The complexities of clinical trial management are intensifying as regulations become more stringent and the demand for compliance grows. Organizations striving for success in this landscape must leverage Clinical Trial Management Systems (CTMS) that not only streamline processes but also ensure adherence to legal and quality standards.
This article delves into ten essential features of CTMS systems that can significantly enhance compliance success, addressing the pressing question:
- How can organizations effectively navigate the intricate web of regulatory requirements while optimizing their clinical research outcomes?
AVS Life Sciences: Comprehensive Regulatory and Quality Solutions for CTMS
stands as a leading service provider committed to ensuring regulatory adherence and quality management within research management systems. With a specialization in the pharmaceutical, biotechnology, and medical device sectors, the company offers tailored solutions that empower clients to navigate the complexities of regulatory landscapes with confidence. Focusing on validation, quality compliance consulting, and engineering assistance, AVS Life Sciences ensures that their ctms systems are not only effective but also compliant with Good Manufacturing Practices (GMP) and ISO standards. This foundational expertise, which includes services in Data Integrity and Animal Test Facilities, is crucial for organizations aiming to enhance their clinical research processes and maintain high-quality standards throughout the product lifecycle.
The global clinical research system market is projected to experience significant growth, with an estimated valuation reaching USD 3.87 billion by 2032. This trend underscores the increasing demand for efficient study oversight solutions. As organizations face mounting pressure to comply with stringent regulations, the role of quality control in clinical trial systems becomes paramount. Implementing effective quality management practices can lead to improved clinical study success rates, ensuring that studies are conducted in alignment with regulatory requirements and best practices.
Recent trends reveal a shift toward cloud-based ctms systems, which provide scalability and accessibility, particularly benefiting smaller institutions that may struggle with the financial burdens of traditional systems. These cloud solutions not only reduce initial costs but also facilitate real-time data access and collaboration among stakeholders, enhancing communication and overall execution.
In this evolving landscape, AVS Life Sciences distinguishes itself through its ability to deliver comprehensive regulatory and quality solutions, supporting clients in achieving compliance and operational excellence in their studies. The company's remarkable 80% repeat business rate serves as a testament to its commitment to client satisfaction and effective service delivery.
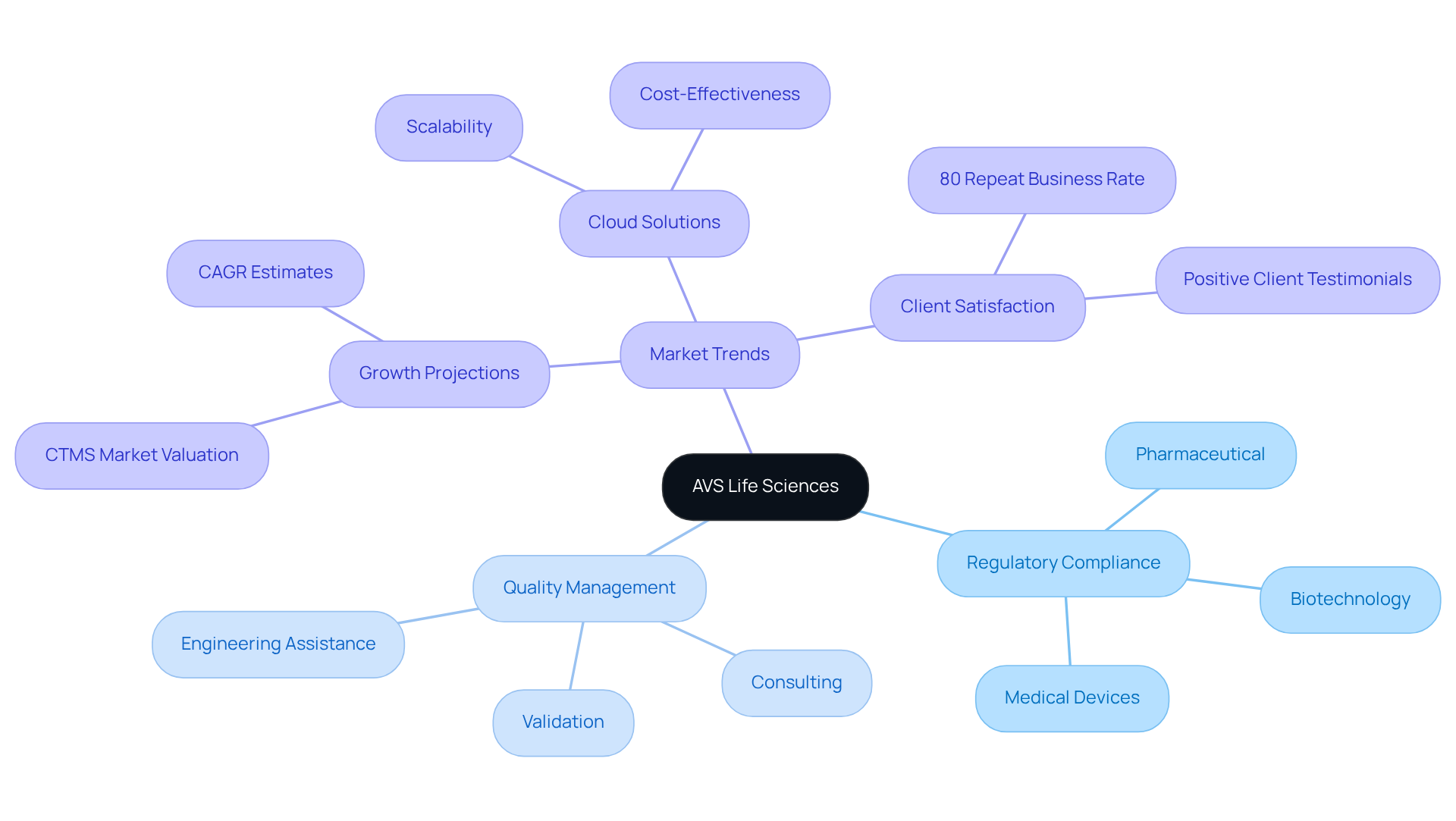
Electronic Data Capture (EDC) Systems: Streamlining Data Management in CTMS
Electronic Data Capture (EDC) systems are essential for enhancing CTMS systems, facilitating the efficient collection, management, and analysis of clinical study data. By replacing traditional paper-based methods, EDC systems significantly reduce errors and enhance data accuracy. They enable real-time data input and validation, ensuring precise data collection at every stage of the experiment. This integration of EDC with CTMS systems , enhances data integrity, and ensures compliance with regulatory standards such as GXP and FDA regulations. Such synergy not only boosts operational efficiency but also empowers timely decision-making based on reliable data.
Furthermore, EDC systems support critical practices such as:
- Data Integrity Deviations
- Investigations
- CAPA
Alongside robust documentation practices that are vital for compliance. As organizations increasingly recognize the advantages of EDC systems, the demand for their integration with CTMS systems is projected to rise, further enhancing the quality and adherence of clinical studies. For expert guidance in navigating these complexities and ensuring adherence to best practices in Computer System Validation (CSV), contact AVS Life Sciences today.
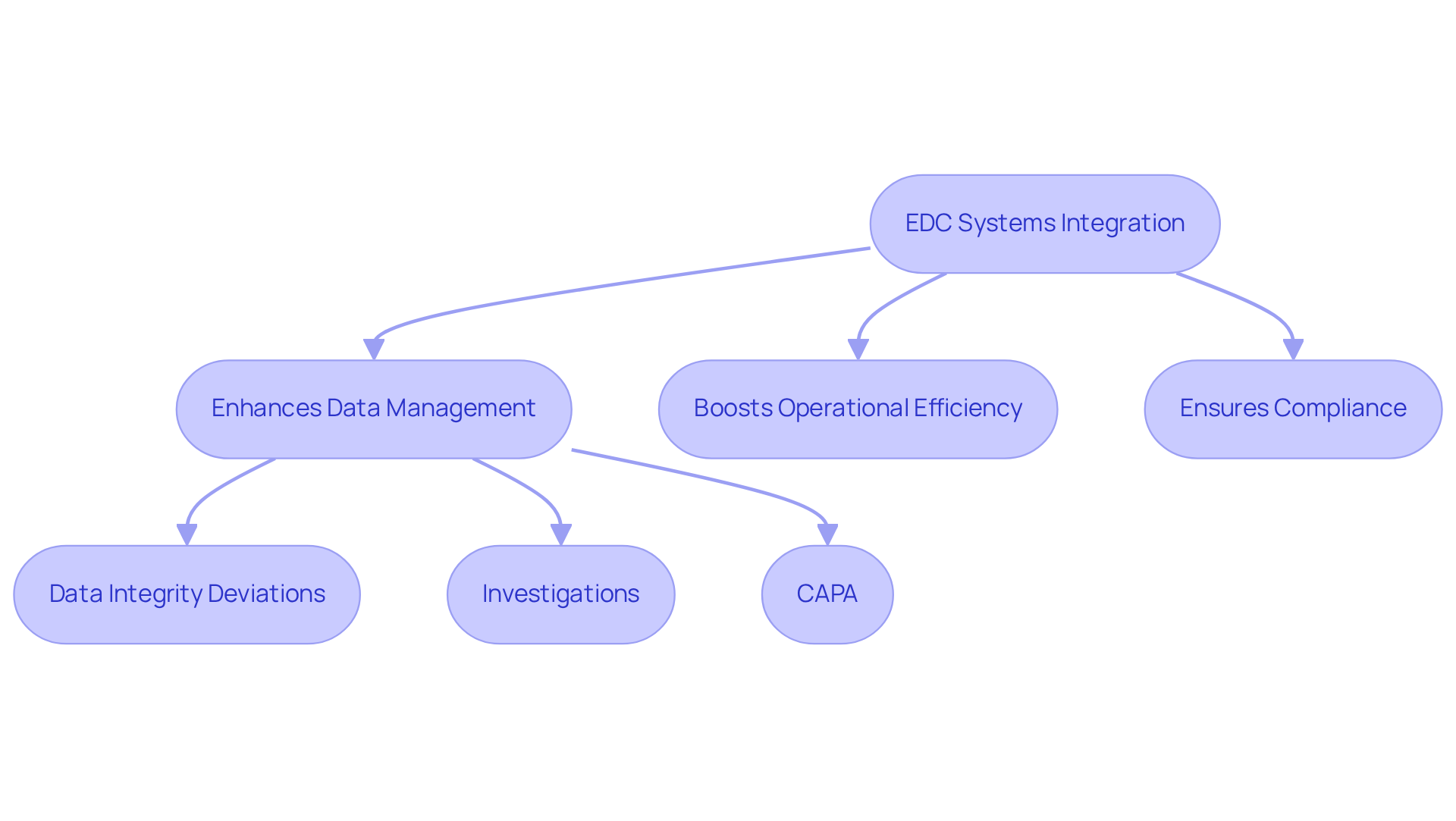
Stakeholder Coordination: Enhancing Collaboration in Clinical Trials
stands as a pivotal element of ctms systems, significantly enhancing collaboration among sponsors, investigators, and regulatory entities. Efficient communication, bolstered by clinical study management systems, streamlines decision-making processes and ensures alignment with the study's objectives, particularly regarding regulatory compliance and quality management practices such as GXP, FDA regulations, and Standard Operating Procedures (SOPs). By managing stakeholder interactions through ctms systems, organizations can mitigate misunderstandings and elevate participant involvement, which is vital for maintaining data integrity and adhering to exemplary documentation practices.
Research indicates that 80% of studies fail to meet enrollment timelines, often due to recruitment challenges, with 50% of sites not achieving their enrollment targets. Furthermore, 40% of research delays are linked to recruitment issues, underscoring the necessity of enhanced stakeholder communication to improve overall study efficiency. As Laura P. Forsythe highlights, most projects engage multiple stakeholders, with 83% of respondents acknowledging interactions with more than one party. By leveraging ctms systems, organizations can cultivate a more unified environment that accelerates the research process and adapts to the evolving needs of all involved parties, thereby ensuring compliance with essential monitoring techniques and GXP sponsor responsibilities.
Scheduling and Oversight: Optimizing Clinical Trial Timelines
Management systems provide robust scheduling and oversight capabilities essential for optimizing research timelines and ensuring compliance with regulatory standards. These systems streamline the planning of study activities, such as patient visits, data collection, and regulatory submissions, while strictly adhering to GXP and FDA regulations. By automating scheduling processes, ctms systems assist organizations in averting bottlenecks and ensuring that all research activities are executed punctually—an absolute necessity in the fast-paced world of medical studies. Furthermore, the integration of comprehensive quality management practices, including:
significantly enhances the reliability of results. This capability is particularly crucial, as delays can lead to , underscoring the importance of effective oversight of service providers for achieving success.
Record Keeping and eTMF: Ensuring Compliance and Documentation
Efficient record maintenance is essential for compliance in clinical studies, with ctms systems playing a pivotal role through the implementation of electronic Master Files (eMF). These systems centralize all trial-related documents, ensuring they are organized and readily accessible. This streamlined method not only simplifies the documentation process but also significantly enhances by providing a clear and comprehensive record of all activities. Maintaining accurate and up-to-date records is crucial, as it allows organizations to demonstrate compliance with regulatory requirements and facilitates smoother inspections.
Recent findings indicate that 77% of observations during audits were related to missing operational records, underscoring the necessity of robust record-keeping practices. By leveraging eTMF systems, organizations can effectively mitigate risks associated with documentation errors and ensure adherence to Good Manufacturing Practices (GMP), ISO standards, and Quality System Regulations (QSR). Moreover, contemporary eTMF applications are designed to be user-friendly and can be pre-configured for quick implementation, often prepared for use within two to three weeks. This capability significantly decreases the time and complexity typically linked with documentation oversight.
In conclusion, the integration of eTMF systems not only addresses compliance challenges but also empowers organizations to enhance their operational efficiency and readiness for regulatory scrutiny. The importance of investing in ctms systems cannot be overstated, as these systems represent a strategic advantage in navigating the complexities of clinical study management.
Financial Agreements and Remuneration: Managing Budgets in CTMS
Management systems are essential for managing financial contracts and compensation, which play a critical role in ensuring budgetary oversight in research studies. These systems enable precise tracking of expenses, , and prompt processing of payments to participants and vendors. By automating financial processes, the system significantly alleviates administrative burdens and mitigates the risk of budget overruns—a common challenge in research, where over 80% of studies encounter delays averaging one to six months, and only 10% are completed on time.
Efficient financial supervision is vital for ensuring that research studies remain economically viable and comply with regulatory standards. Moreover, integrating financial oversight tools within clinical systems can lead to more accurate budget forecasts, empowering organizations to navigate the complexities of clinical study funding with greater efficacy.
As discussions around budget overruns evolve towards optimization strategies, it becomes increasingly imperative to leverage ctms systems for budget oversight to ensure effective study coordination. Notably, final budgets often exceed initial proposals by more than 50%, and incomplete studies may require additional funding exceeding AUD$2 million (260% above budget), underscoring the financial risks associated with managing these processes.
As Chris Schilling observed, the repercussions of overestimating recruitment rates can be substantial, further highlighting the financial consequences of inadequate budget management.
Participant Monitoring and EDC Synchronization: Ensuring Data Integrity
Participant oversight stands as a cornerstone of ctms systems, which are crucial for safeguarding the integrity of data collected during research studies. By synchronizing Electronic Data Capture (EDC) systems with participant monitoring tools, organizations can effectively track patient progress, adherence to protocols, and any adverse events in real-time. This integration not only facilitates immediate data validation and correction but also significantly mitigates the risk of errors, thereby enhancing the overall quality of the data gathered.
Effective participant monitoring using ctms systems ensures compliance with regulatory standards, including GXP and FDA regulations, while prioritizing the safety and well-being of study participants. As Meri Beckwith aptly notes, 'Continuous statistical analyses can identify adverse events or trends that necessitate changes to study protocols or participant inclusion criteria.'
Through the utilization of , organizations can establish a more robust data handling framework, ultimately leading to improved outcomes in research. [AVS Life Sciences, a leading provider of quality management and regulatory compliance solutions in the life sciences sector](https://avslifesciences.com/case-studies/fill-finish), exemplifies the effectiveness of managing compliance-driven projects, underscoring the critical role of participant monitoring in clinical studies.
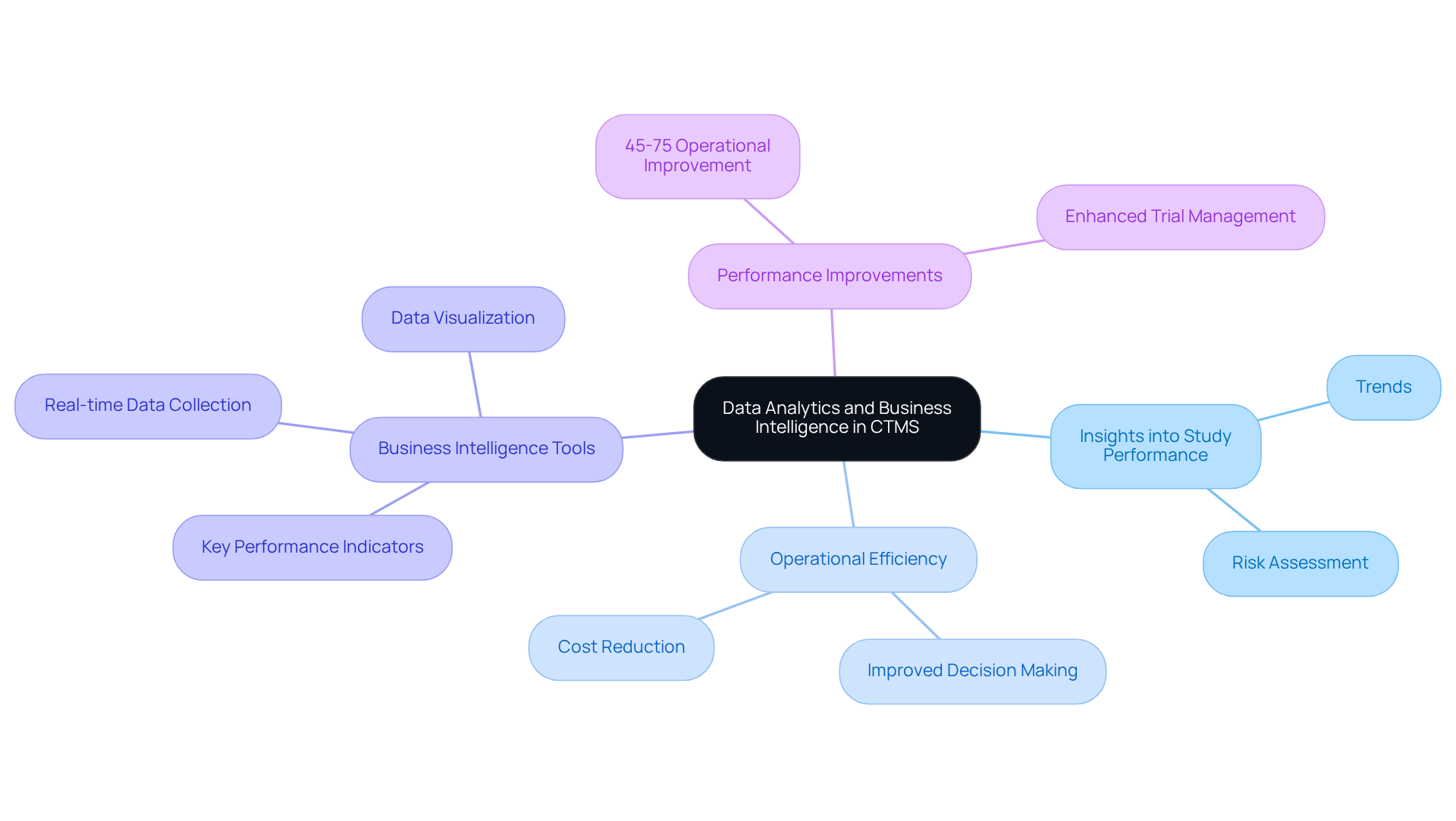
Data Analytics and Business Intelligence: Leveraging Insights in CTMS
Data analytics and business intelligence serve as pivotal components of modern CTMS systems, providing organizations with invaluable insights into study performance and outcomes. By scrutinizing the data collected during experiments, stakeholders can identify trends, assess risks, and make informed decisions that enhance operational efficiency.
Business intelligence tools integrated within CTMS systems visualize intricate data sets, empowering teams to track key performance indicators and adjust strategies as necessary. This data-centric approach not only optimizes trial management but also supports compliance with by ensuring decisions are based on accurate and timely information.
Over the next decade, operational performance improvements could soar by 45-75% with the widespread adoption of data-driven technologies, underscoring the substantial advantages these tools offer. As Guillaume Gigon noted, real-time data collection and analysis are rapidly becoming the norm for study sponsors, further underscoring the critical role of business intelligence in enhancing study outcomes.
A relevant case study, 'Data Driven Trial Design and Decision Making,' exemplifies how business intelligence tools lead to more successful trials by enabling proactive adjustments informed by predictive analytics. By leveraging these insights, organizations can streamline processes and mitigate the risk of costly delays, ultimately resulting in more successful clinical studies.
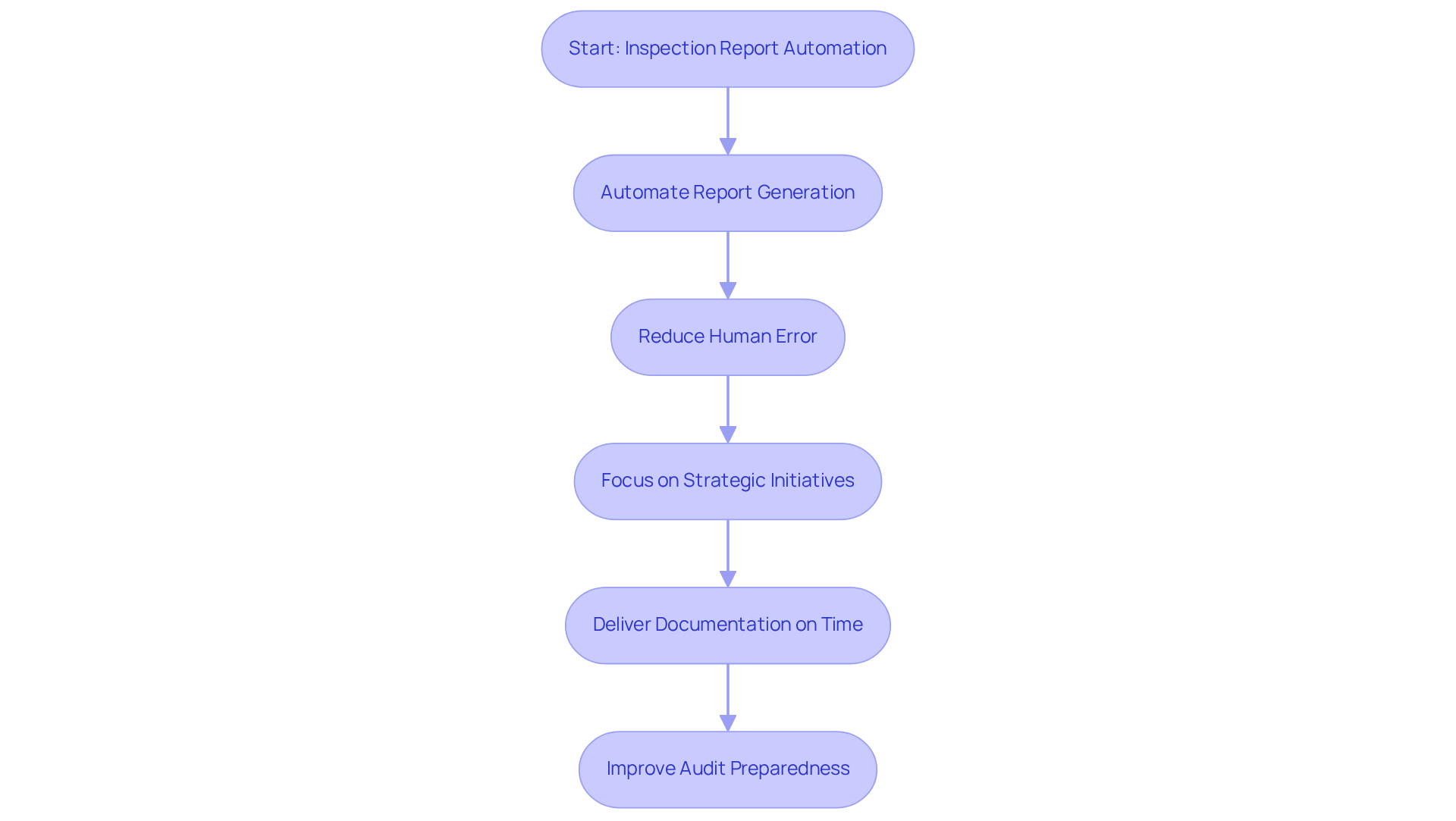
Inspection Report Creation and Automation: Streamlining Compliance Processes
Automated inspection report generation stands as a pivotal element of compliance procedures, significantly bolstering audit preparedness. By automating the creation of inspection reports, organizations can guarantee that all necessary documentation is meticulously prepared and delivered without delay. , allowing teams to concentrate on strategic initiatives, such as tackling compliance challenges and refining research methodologies. Furthermore, well-organized and readily accessible inspection reports facilitate smoother interactions with regulatory authorities during audits and inspections, ultimately cultivating a more efficient compliance landscape.
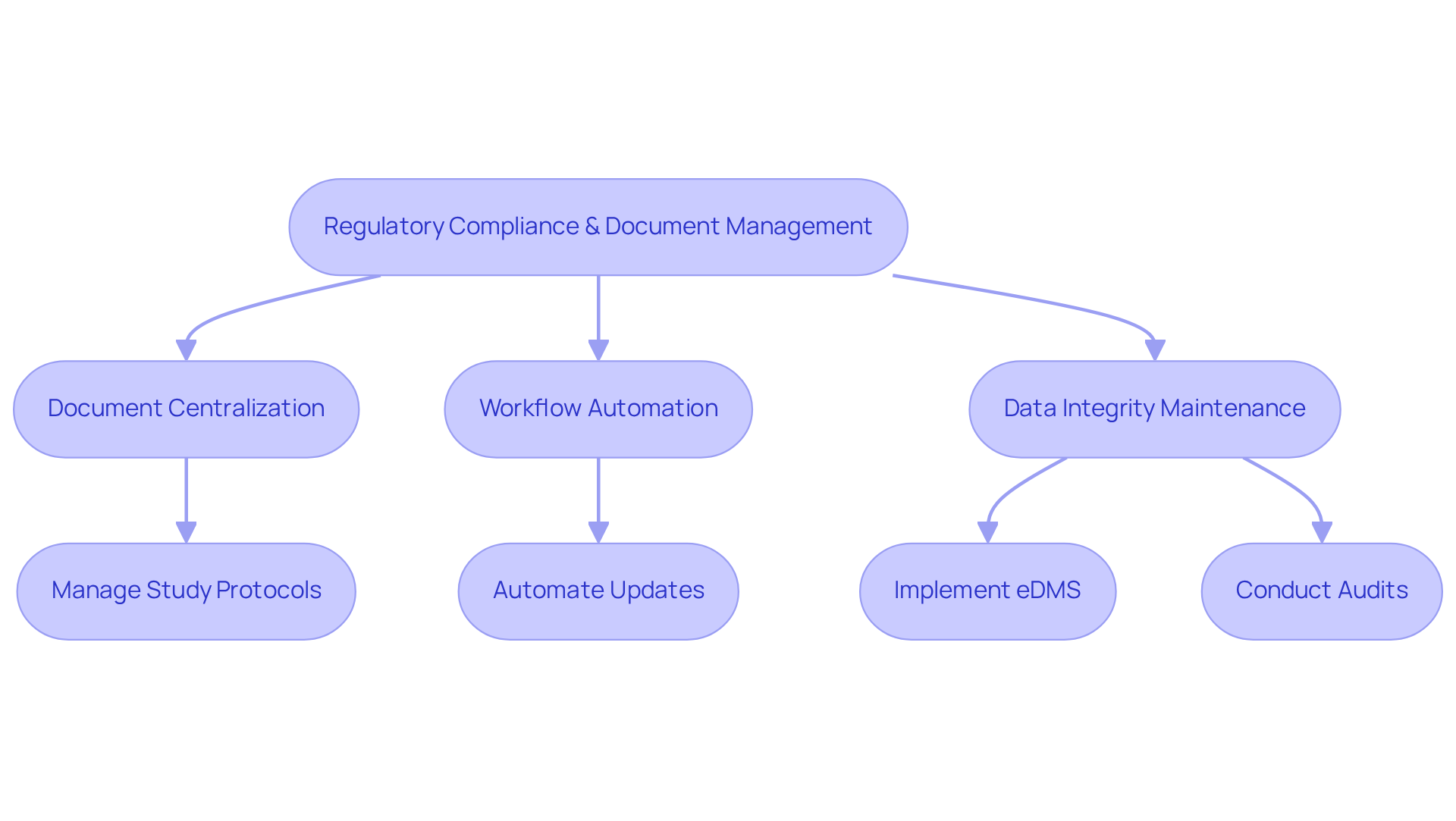
Regulatory Compliance and Document Management: Upholding Legal Standards in CTMS
Regulatory compliance and document oversight are critical components of Clinical Study Systems, ensuring adherence to legal standards throughout the research process. CTMS systems offer comprehensive tools for managing essential documents, such as study protocols, informed consent forms, and regulatory submissions. By centralizing these documents and automating workflows, organizations can effectively maintain compliance with regulatory requirements, including GXP and FDA regulations, while facilitating timely updates and revisions as necessary. This robust document organization feature not only bolsters but also significantly enhances the overall efficiency of research operations.
For instance, research studies that utilize integrated, automated data handling workflows have been shown to finalize patient enrollment 30% faster and achieve database lock in 45% less time compared to those relying on fragmented systems. Furthermore, the implementation of electronic document handling systems (eDMS) can reduce data entry errors by 40%, underscoring the importance of efficient document oversight in maintaining data integrity and compliance. Adhering to standard operating procedures (SOPs) and ensuring version control is essential for maintaining accurate records of all modifications to documents in clinical studies.
Additionally, internal and external auditing techniques further reinforce compliance by providing oversight and accountability. As organizations navigate the complexities of regulatory frameworks, leveraging CTMS systems for document management becomes indispensable for safeguarding trial integrity and ensuring successful outcomes.
Conclusion
The exploration of essential features within Clinical Trial Management Systems (CTMS) underscores the critical role these tools play in ensuring compliance and enhancing operational efficiency in clinical research. By integrating various functionalities, organizations can adeptly navigate the complexities of regulatory landscapes while upholding high standards of quality throughout the study lifecycle.
Key insights from the article emphasize the necessity of robust systems for data management, stakeholder coordination, and financial oversight. The integration of Electronic Data Capture (EDC) systems, effective scheduling, and comprehensive record-keeping practices are vital for streamlining processes and mitigating risks associated with compliance failures. Furthermore, the focus on data analytics and automated report generation illustrates how technology can drive informed decision-making and bolster audit readiness.
Ultimately, investing in advanced CTMS solutions transcends mere strategic advantage; it is a necessity for organizations striving to excel in the competitive landscape of clinical research. By prioritizing compliance and operational excellence, stakeholders can ensure successful study outcomes, safeguard participant safety, and uphold the integrity of the research process. Embracing these essential features will pave the way for a more efficient and compliant future in clinical trials, ultimately benefiting the broader healthcare landscape.
Frequently Asked Questions
What services does AVS Life Sciences provide?
AVS Life Sciences offers comprehensive regulatory adherence and quality management solutions tailored for the pharmaceutical, biotechnology, and medical device sectors, focusing on validation, quality compliance consulting, and engineering assistance.
How does AVS Life Sciences ensure compliance with regulatory standards?
The company ensures compliance with Good Manufacturing Practices (GMP) and ISO standards, providing foundational expertise in areas such as Data Integrity and Animal Test Facilities to enhance clinical research processes.
What is the projected growth of the global clinical research system market?
The global clinical research system market is projected to reach an estimated valuation of USD 3.87 billion by 2032, indicating significant growth and increasing demand for efficient study oversight solutions.
What trends are emerging in clinical trial management systems (CTMS)?
There is a notable shift towards cloud-based CTMS systems, which offer scalability and accessibility, particularly benefiting smaller institutions by reducing initial costs and enhancing real-time data access and collaboration.
What is the significance of Electronic Data Capture (EDC) systems in CTMS?
EDC systems enhance CTMS by facilitating efficient collection, management, and analysis of clinical study data, reducing errors, ensuring data accuracy, and streamlining workflows while ensuring compliance with regulatory standards.
What practices do EDC systems support to ensure compliance?
EDC systems support critical practices such as Data Integrity Deviations, Investigations, and Corrective and Preventive Actions (CAPA), along with robust documentation practices essential for compliance.
Why is stakeholder coordination important in clinical trials?
Stakeholder coordination enhances collaboration among sponsors, investigators, and regulatory entities, streamlining decision-making and ensuring alignment with study objectives, particularly regarding regulatory compliance and quality management.
What challenges are associated with study enrollment in clinical trials?
Research indicates that 80% of studies fail to meet enrollment timelines due to recruitment challenges, with 50% of sites not achieving their enrollment targets, highlighting the need for improved stakeholder communication.
How can CTMS systems improve study efficiency?
By managing stakeholder interactions and enhancing communication through CTMS systems, organizations can mitigate misunderstandings, elevate participant involvement, and adapt to the evolving needs of all parties involved in the research process.
