10 Essential Compliance Training Courses for Pharmaceutical Officers

Overview
The article outlines ten essential compliance training courses for pharmaceutical officers, emphasizing critical areas such as Good Manufacturing Practices (GMP), regulatory requirements, and ethical standards in clinical research. These courses are meticulously designed to equip professionals with the vital knowledge and skills necessary to navigate the complex regulatory landscapes. By enhancing adherence and operational excellence within the pharmaceutical industry, these training programs address the pressing compliance challenges faced by professionals today. Engaging with these courses not only fosters individual growth but also strengthens the overall integrity of the industry.
Introduction
The pharmaceutical industry operates in a landscape defined by stringent regulations and evolving compliance standards. As professionals navigate this intricate terrain, the necessity for specialized training becomes paramount. This article delves into ten essential compliance training courses specifically tailored for pharmaceutical officers, aimed at equipping them with the knowledge and skills essential for upholding regulatory adherence and enhancing operational efficiency. With a plethora of options available, the critical question arises: how can one discern which training programs genuinely provide the insights necessary to confront the industry's complex challenges?
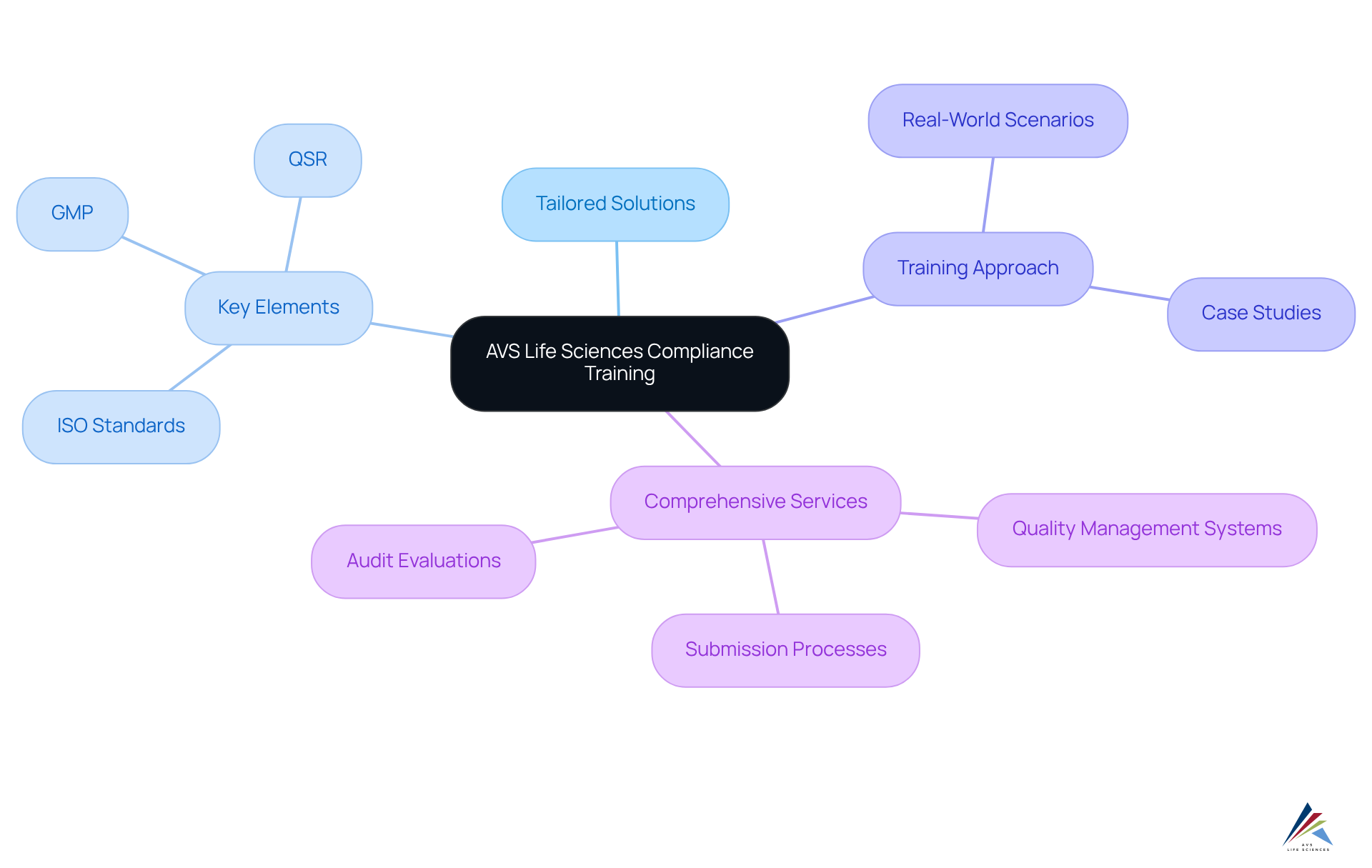
AVS Life Sciences Compliance Training: Tailored Solutions for Regulatory Adherence
AVS Life Sciences delivers tailored adherence development programs specifically designed for pharmaceutical officers, addressing crucial elements such as Good Manufacturing Practices (GMP), Quality System Regulations (QSR), and ISO standards. These programs incorporate real-world scenarios and case studies, including our successful upgrade of a biotechnology client's GMP facility from Level 1 to Level 2, equipping participants with practical insights directly applicable to their roles. This approach not only enhances their understanding of legal obligations but also significantly strengthens their ability to maintain adherence throughout the product lifecycle.
The effectiveness of these development programs is underscored by the latest trends in adherence education, which emphasize the need for customized learning experiences that adapt to the evolving legal landscape. As organizations increasingly recognize the importance of tailored education, AVS Life Sciences remains at the forefront, ensuring that pharmaceutical officers possess the knowledge and skills necessary to adeptly navigate complex regulatory challenges. Our comprehensive services include:
- Quality Management Systems development
- Submission processes
- Audit evaluations
All crafted to empower pharmaceutical officers to uphold the highest standards of regulatory adherence.
Regulatory Affairs Professionals Society (RAPS) Compliance Training: Focused on Pharmaceutical Regulations
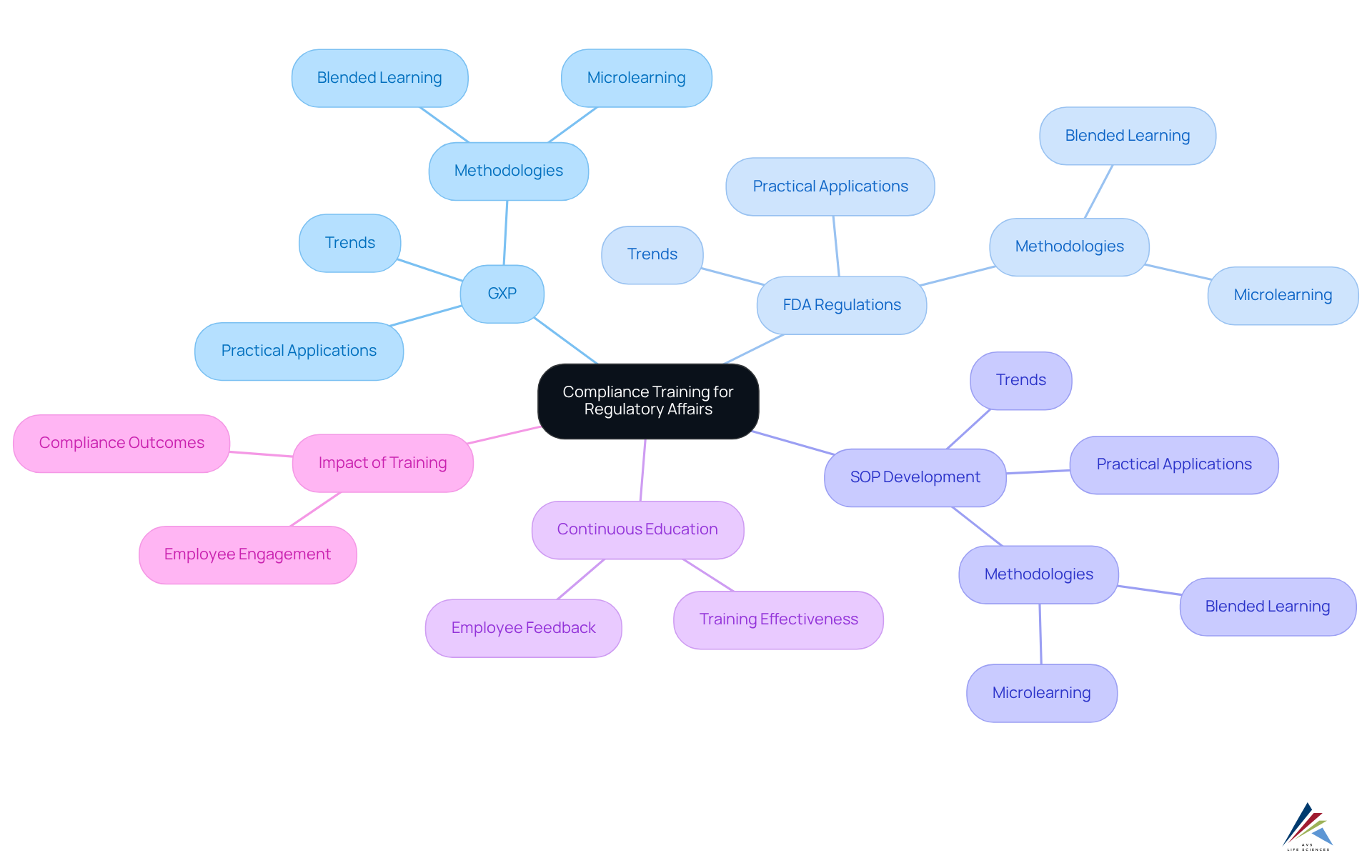
AVS Life Sciences offers a robust suite of consulting services and educational programs meticulously designed for regulatory affairs professionals within the biopharmaceutical sector. These compliance training courses emphasize critical areas such as GXP, FDA regulations, and the development of Standard Operating Procedures (SOPs), ensuring adherence to international regulatory standards.
By engaging in these comprehensive compliance training courses, professionals are equipped to navigate the complexities of regulations, ultimately enhancing their contributions to their organizations. The emphasis on practical application in compliance training courses ensures that learners can effectively implement their knowledge in real-world contexts, which improves compliance outcomes in the pharmaceutical industry.
A compelling case study exemplifies AVS Life Sciences' successful optimization of a biotechnology GMP facility, where our documentation efforts demonstrated complete traceability and adherence to assurance standards. This collaboration enabled our client to concentrate on developing therapies that enhance patient quality of life.
Current trends reveal an increasing demand for specialized education, with organizations acknowledging the importance of compliance training courses to remain abreast of evolving regulations and best practices in drug approval processes. Furthermore, statistics show that only 10% of employees report that adherence education has significantly impacted their work practices, underscoring the critical need for effective compliance training courses like those offered by AVS Life Sciences.
Continuous education and employee feedback are essential components in assessing the quality of instruction, ensuring it aligns with the dynamic demands of the industry. The integration of diverse modalities, such as blended learning and microlearning, can further bolster the effectiveness of compliance training courses, making them an indispensable investment for organizations striving to uphold high standards in regulatory affairs.
Drug Enforcement Administration (DEA) Training: Essential Knowledge on Controlled Substances
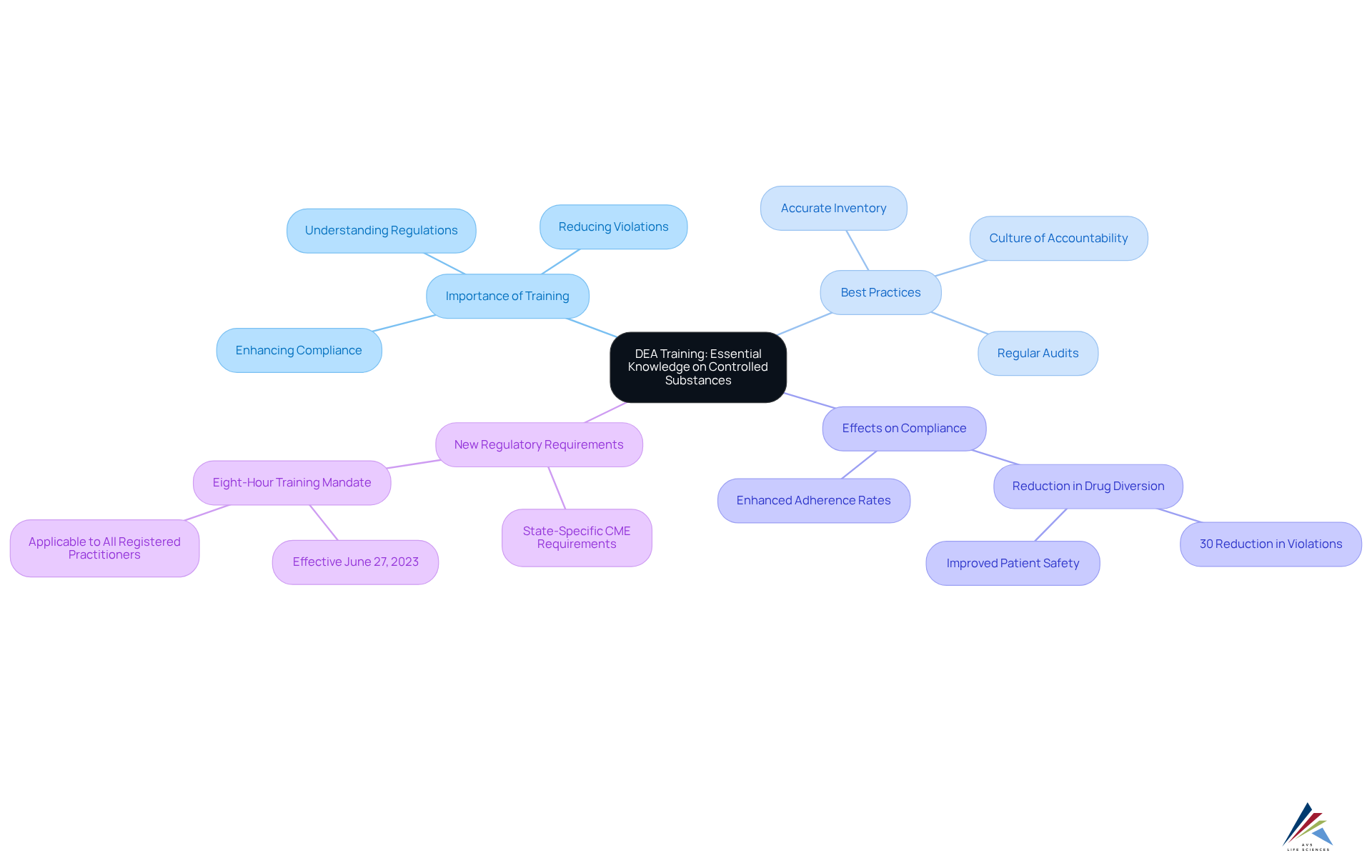
Drug Enforcement Administration (DEA) Training: Essential Knowledge on Controlled Substances
DEA training is pivotal for understanding the regulations governing controlled substances, encompassing proper handling, meticulous recordkeeping, and stringent reporting requirements. This program is not merely a formality; it equips professionals with the knowledge necessary to navigate the complex legal landscape associated with these substances. By participating in this program, individuals can significantly reduce the likelihood of violations, as evidenced by enhanced adherence rates in organizations that have adopted such initiatives.
For instance, healthcare institutions that implemented thorough adherence education reported a notable decline in drug diversion occurrences, with some achieving a reduction in violations by over 30%. Best practices emphasized in the training include:
- Maintaining accurate inventory records
- Conducting regular audits
- Fostering a culture of accountability among staff
These measures not only improve adherence but also protect patient safety and uphold the integrity of healthcare systems. As the landscape of pharmaceutical regulations continues to evolve, compliance training courses and adherence to DEA guidelines remain essential for compliance officers in the pharmaceutical industry.
Importantly, the new DEA requirement mandates that all registered practitioners complete a one-time, eight-hour course on the treatment and management of patients with opioid or other substance use disorders, effective June 27, 2023. This underscores the urgency and significance of compliance training courses in the current oversight landscape.
AVS Life Sciences offers comprehensive management education programs that equip professionals with the essential skills and knowledge to address these changing legal requirements.
Society of Clinical Research Associates (SoCRA) Training: Ethical Compliance in Clinical Research
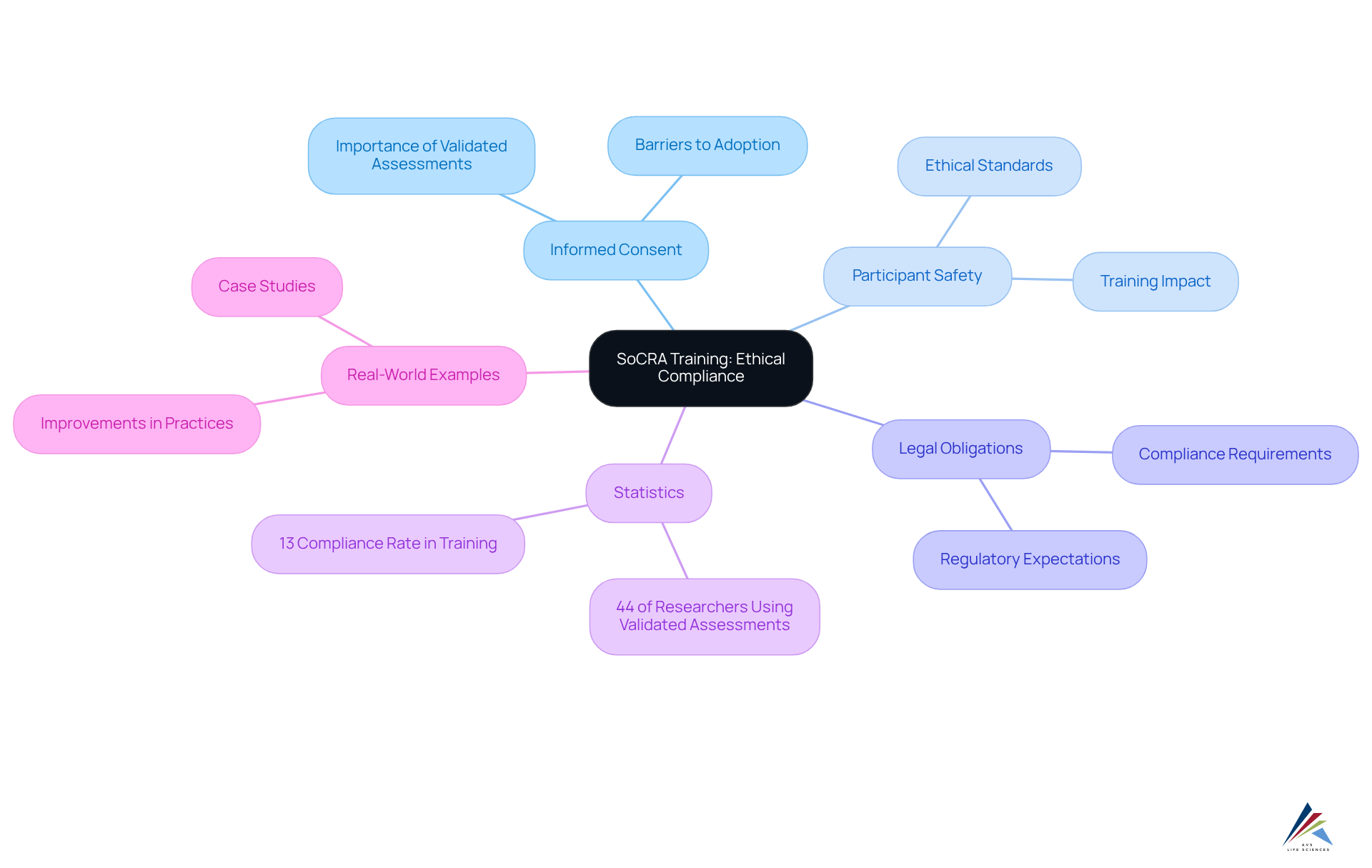
SoCRA offers extensive educational programs that underscore the importance of ethical adherence in clinical research, focusing on critical elements such as informed consent, participant safety, and legal obligations. These compliance training courses are meticulously designed to enhance professionals' understanding of ethical standards and ensure their research practices align with regulatory expectations. By engaging in SoCRA education, individuals not only elevate their comprehension but also bolster the integrity of clinical trials, fostering a culture of adherence that prioritizes participant welfare.
Statistics reveal a concerning gap: only 44% of researchers employ validated assessments of consent, underscoring the necessity of SoCRA education in rectifying such deficiencies. As Kari A Baldwin asserts, "Resources and education to enhance awareness and understanding of LARs are necessary," emphasizing the pivotal role of instruction in ethical adherence.
Real-world examples further illustrate the efficacy of this instruction, showcasing improved informed consent practices and heightened awareness of ethical considerations among clinical research professionals. For instance, a study examining the adoption of validated assessments of consent understanding found that merely 13% of participants completed the preparation in a reasonable timeframe, signaling ongoing challenges in adherence. As the landscape of clinical trials evolves, the importance of compliance training courses in maintaining high ethical standards cannot be overstated.
International Society for Pharmaceutical Engineering (ISPE) GMP Training: Ensuring Manufacturing Compliance
The International Society for Pharmaceutical Engineering (ISPE) offers a robust GMP educational program that delves into the essential principles and practices crucial for compliance training courses in pharmaceutical manufacturing. Participants engage with critical topics such as:
- Systems
- GXP
- FDA guidelines
- Risk management in compliance training courses
Gaining insights into compliance expectations vital for implementing effective assurance measures. This compliance training course is indispensable for ensuring that manufacturing processes uphold the highest standards of excellence and adherence, fostering a culture of continual improvement.
A pertinent example is AVS Life Sciences' recent partnership with a leading biotechnology company, where they adeptly transitioned a manufacturing facility from a Biosafety Level 1 to a Level 2 GMP facility for lentivirus production. This initiative not only adhered to stringent regulatory compliance but also underscored the importance of assurance and meticulous documentation practices throughout the transition.
As industry leaders emphasize, achieving excellence in standards is not merely an objective but a practice cultivated through ongoing education and compliance training courses. The latest GMP development programs for 2025 are designed to equip pharmaceutical professionals with the skills necessary to navigate the complexities of modern manufacturing environments, highlighting the critical role of robust assurance systems in mitigating risks and enhancing overall product integrity.
To maximize the benefits of this course, regulatory officers should actively apply the concepts learned in their daily operations, ensuring that quality assurance measures are not just theoretical but also practical and effective.
Food and Drug Administration (FDA) Training: Navigating Regulatory Requirements
FDA training programs are crucial for understanding the intricate guidelines governing pharmaceuticals, particularly regarding drug approval processes, labeling, and post-market surveillance. These compliance training courses provide experts with invaluable insights into the evolving legal landscape, enabling them to tackle compliance challenges effectively.
For example, the Pharmaceutical Quality Symposium 2023 highlighted essential quality evaluation principles and cutting-edge manufacturing technologies, showcasing how compliance science evolves in response to new challenges in pharmaceutical development. Similarly, the Generic Drugs Forum (GDF) has focused on enhancing access to generic medications through increased awareness of guidelines, illustrating the tangible impact of FDA training on adherence success.
Current trends reveal a heightened focus on compliance training courses that integrate regulatory science with practical implementation, ensuring that professionals are thoroughly equipped to navigate the complexities of drug approval processes. By engaging in these development opportunities, pharmaceutical professionals can significantly enhance their adherence capabilities, ultimately leading to more efficient and successful drug approval outcomes.
American Society for Quality (ASQ) Training: Mastering Quality Management Systems
The American Society for Excellence (ASQ) provides comprehensive educational programs focused on the fundamentals of management systems, which include essential components such as planning, control, and enhancement. Participants gain invaluable insights into the implementation of effective management practices through compliance training courses that not only meet regulatory requirements but also significantly improve adherence and product excellence within their organizations. This development is particularly vital for professionals aiming to advance their careers in standards management, especially in 2025, as the sector faces increased scrutiny and evolving regulations.
Notably, firms that invest in management training have achieved remarkable results; for instance, organizations that adopted role-specific qualification programs experienced a 41% reduction in regulatory errors. A compelling case study from AVS Life Sciences underscores this point: a leading biotechnology company successfully upgraded its GMP facility with AVS's support, ensuring compliance and operational excellence. AVS employed a systematic approach that included thorough gap analysis, meticulous planning, and stringent assurance protocols throughout the upgrade process. This collaboration allowed the client to focus on drug development while AVS managed the complexities of the upgrade, including addressing assurance challenges.
Furthermore, as W. Edwards Deming aptly stated, 'Excellence arises not from inspection, but from enhancement of the production process,' emphasizing the necessity of embedding management principles into everyday practices. By prioritizing compliance training courses in quality planning and control education, pharmaceutical professionals can equip themselves to adeptly navigate the intricacies of regulations. To fully leverage the benefits of such development, professionals should actively engage in role-specific programs that align with their organizational goals.
Compliance Certification Board (CCB) Training: Best Practices for Compliance Professionals
CCB instruction is crucial for professionals navigating the complexities of adherence, focusing on best practices in risk management, audits, and policy updates. This program equips participants with a profound understanding of regulatory frameworks, enabling them to develop effective adherence initiatives tailored for the pharmaceutical industry.
Organizations that prioritize adherence education have reported significant improvements in audit results, with numerous entities achieving zero findings during regulatory evaluations. Furthermore, a proactive approach to regulatory education fosters a culture of accountability, empowering employees to identify and mitigate risks early.
By enhancing their capabilities through CCB instruction, regulatory experts can ensure their organizations maintain the highest standards, ultimately safeguarding against costly fines and reputational damage.
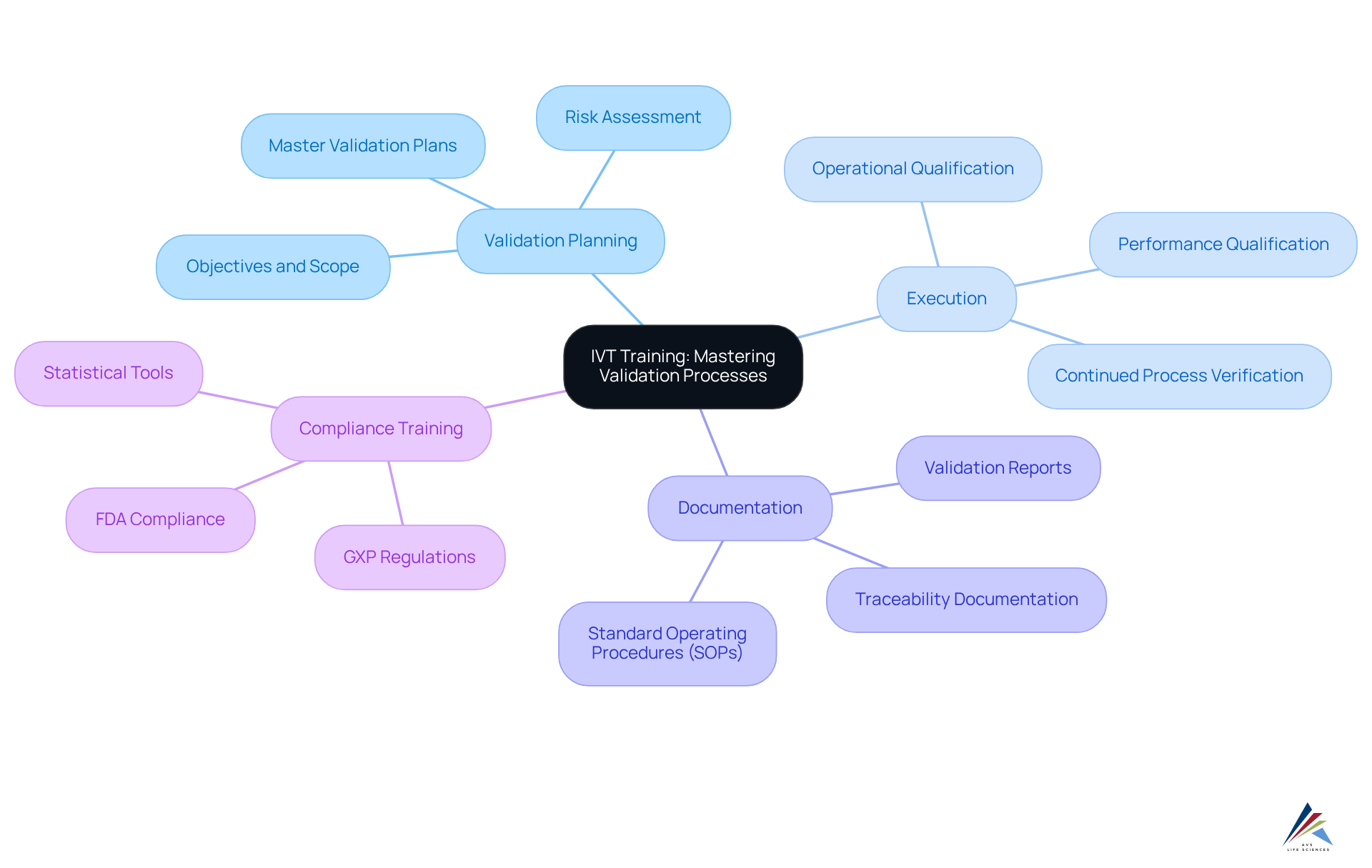
Institute of Validation Technology (IVT) Training: Mastering Validation Processes
AVS Life Sciences delivers specialized development programs focused on validation processes within the pharmaceutical sector. These courses address critical topics such as validation planning, execution, and documentation, including essential documents like SOPs, master validation plans, and validation reports. Engaging in compliance training courses allows professionals to enhance their understanding of validation requirements, including GXP and FDA regulations, ensuring alignment with regulatory standards and ultimately contributing to product excellence.
Practical examples underscore the effectiveness of validation instruction in fostering adherence. For instance, AVS Life Sciences recently supported a leading biotechnology company in upgrading its manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. This project was completed on time and within budget, showcasing the success of structured validation planning. The documentation efforts, which ensured full traceability, were deemed suitable by the client's assurance team, resulting in smoother audits and fewer compliance-related issues.
As emphasized by industry experts, effective validation instruction transcends mere education; it represents a strategic investment in upholding high standards of quality and compliance. Notably, 70% of companies have reported delays in product launches due to validation issues, highlighting the urgent need for robust development programs.
Quotes from influential figures in the field further accentuate the importance of this instruction. One expert stated, 'The commitment to using statistical tools reflects a broader dedication to process excellence, setting companies apart in a competitive industry.' Additionally, Ershad Moradi noted, 'Utilizing statistical tools enables organizations to create high-quality products, reduce costs, and uphold legal standards efficiently.' This sentiment encapsulates the essence of AVS Life Sciences' development programs, which empower professionals to maintain stringent regulatory standards through informed validation practices, with a strong emphasis on Data Integrity and Technical Writing.
Association of Clinical Research Organizations (ACRO) Training: Compliance in Clinical Trials
AVS Life Sciences offers comprehensive educational programs that highlight the importance of adherence in clinical trials, addressing critical topics such as legal obligations, ethical considerations, and effective trial management strategies. A key aspect of these programs is the in-depth exploration of the computer system validation (CSV) process, which is essential for ensuring compliance within the life sciences sector. The CSV process, as detailed in the Good Automated Manufacturing Practices (GAMP) 5 Guide, encompasses several crucial stages:
- Planning: Management outlines the required budget and timeline for computer system validation.
- Defining User Requirement Specifications (URS): This stage involves identifying the tasks that a system must perform.
- Design Specifications: The team determines the design and functionality to meet established requirements.
- Building and Configuring a System: Development of configured scripts for software implementation occurs here.
- Installation Qualification (IQ) Testing: Testing is conducted to confirm the correct installation method is utilized.
- Operational Qualification (OQ) Testing: This verifies that the system functions as intended within the user environment.
- Performance Qualification (PQ) Testing: Simulations of worst-case scenarios are performed to ensure ongoing functionality.
- Reporting: Documentation of all actions taken during IQ, OQ, and PQ testing is completed.
By participating in compliance training courses provided by AVS Life Sciences, professionals can enhance their understanding of these standards, ensuring that their clinical trials adhere to ethical guidelines and legal requirements. This expertise ultimately contributes to the success of their research endeavors, establishing AVS Life Sciences as a trusted partner for dependable consulting in quality management and regulatory compliance.

Conclusion
The significance of compliance training for pharmaceutical officers is paramount, as it lays a crucial foundation for navigating the intricate regulatory landscape of the industry. Engaging in tailored training programs not only deepens professionals' understanding of essential regulations but also equips them with the skills necessary to maintain adherence throughout the product lifecycle, ensuring both safety and efficacy in pharmaceutical practices.
This article has spotlighted various training programs, each focusing on distinct aspects of compliance. From AVS Life Sciences’ customized solutions addressing Good Manufacturing Practices (GMP) to the ethical standards promoted by the Society of Clinical Research Associates (SoCRA), these courses are crafted to meet the evolving demands of the industry. Moreover, the importance of programs such as the Drug Enforcement Administration (DEA) training, which underscores the proper handling of controlled substances, further illustrates the diverse range of topics encompassed by compliance training.
As the pharmaceutical industry grapples with increasing scrutiny and regulatory changes, investing in compliance training is not merely advantageous but essential. Organizations must prioritize these educational initiatives to cultivate a culture of accountability and excellence, ultimately leading to improved compliance outcomes and enhanced patient safety. By championing continuous education and adapting to the latest trends, pharmaceutical officers can ensure they are well-prepared to confront future challenges and uphold the highest standards of regulatory adherence.
Frequently Asked Questions
What types of compliance training does AVS Life Sciences offer?
AVS Life Sciences offers tailored adherence development programs for pharmaceutical officers, focusing on Good Manufacturing Practices (GMP), Quality System Regulations (QSR), ISO standards, GXP, FDA regulations, and the development of Standard Operating Procedures (SOPs).
How does AVS Life Sciences ensure the effectiveness of its training programs?
The effectiveness of AVS Life Sciences' training programs is enhanced through real-world scenarios, case studies, and a focus on practical application, which helps participants understand legal obligations and improve compliance outcomes.
Can you provide an example of a successful project by AVS Life Sciences?
One notable example is the upgrade of a biotechnology client's GMP facility from Level 1 to Level 2, which demonstrated the effectiveness of their training programs in achieving regulatory compliance.
What are the current trends in compliance training according to AVS Life Sciences?
Current trends highlight the increasing demand for specialized, customized education that adapts to evolving regulations and emphasizes continuous education and employee feedback to ensure quality instruction.
What is the importance of DEA training, and what does it cover?
DEA training is crucial for understanding regulations regarding controlled substances, including proper handling, recordkeeping, and reporting requirements. It helps reduce violations and enhances adherence rates in organizations.
What new requirement has been introduced by the DEA regarding training?
Effective June 27, 2023, all registered practitioners are required to complete a one-time, eight-hour course on the treatment and management of patients with opioid or other substance use disorders.
How does AVS Life Sciences support compliance officers in the pharmaceutical industry?
AVS Life Sciences provides comprehensive management education programs that equip professionals with the necessary skills and knowledge to navigate changing legal requirements and uphold high standards of regulatory adherence.
