10 Essential Clinical Trial Management Software Solutions
Overview
This article identifies ten essential clinical trial management software (CTMS) solutions that significantly enhance operational efficiency and compliance in clinical research. The software, including AVS Life Sciences, OnCore, and RealTime CTMS, is highlighted for its unique features and capabilities.
These solutions:
- Streamline processes
- Improve data accuracy
- Adapt to evolving regulatory requirements
Thereby supporting the increasing demand for effective management tools in the pharmaceutical sector. By addressing the compliance challenges faced in clinical research, these software solutions not only provide detailed answers but also foster engagement with AVS Life Sciences, ensuring that stakeholders are well-equipped to navigate the complexities of regulatory compliance.
Introduction
As the landscape of clinical research evolves, the demand for effective management solutions has surged. The clinical trial management software (CTMS) market is projected to grow significantly in the coming years. These innovative tools are not merely about compliance; they provide organizations with opportunities to enhance operational efficiency, streamline workflows, and improve data accuracy.
However, with numerous options available, how can research teams identify the best CTMS solutions that truly meet their unique needs and regulatory challenges? This article explores ten essential clinical trial management software solutions, highlighting their key features and benefits, as well as the transformative impact they can have on the research process.
AVS Life Sciences CTMS: Comprehensive Quality and Regulatory Compliance Solutions
AVS Life Sciences offers a comprehensive clinical trial management software that seamlessly integrates quality oversight and regulatory adherence solutions tailored for the pharmaceutical and biotechnology sectors. This system is pivotal in streamlining testing processes while ensuring strict compliance with Good Manufacturing Practices (GMP) and Quality System Regulations (QSR). By empowering clients throughout the product lifecycle, AVS Life Sciences equips organizations with essential tools to effectively navigate complex regulatory landscapes. This commitment to quality oversight not only enhances operational efficiency but also significantly improves success rates in research studies, establishing AVS as a trusted partner for organizations striving for excellence in their research endeavors.
Recent trends indicate that the market for clinical trial management software (CTMS) is projected to grow at a CAGR of 14.18% from 2024 to 2032, driven by the increasing demand for innovative pharmaceuticals and the need for efficient tools. The U.S. healthcare study oversight system market was valued at USD 0.78 billion in 2023, highlighting substantial growth potential. Case studies have shown that integrating quality control systems within research studies can lead to significant improvements in data accuracy and study effectiveness. For instance, studies utilizing automated data processing workflows have demonstrated that patient enrollment can be completed 30% faster and database lock achieved in 45% less time compared to those relying on fragmented systems, as noted in the Clinical Trials Efficiency Report. As the industry progresses, AVS Life Sciences remains at the forefront, continuously adapting its solutions to meet evolving regulatory requirements and technological advancements.
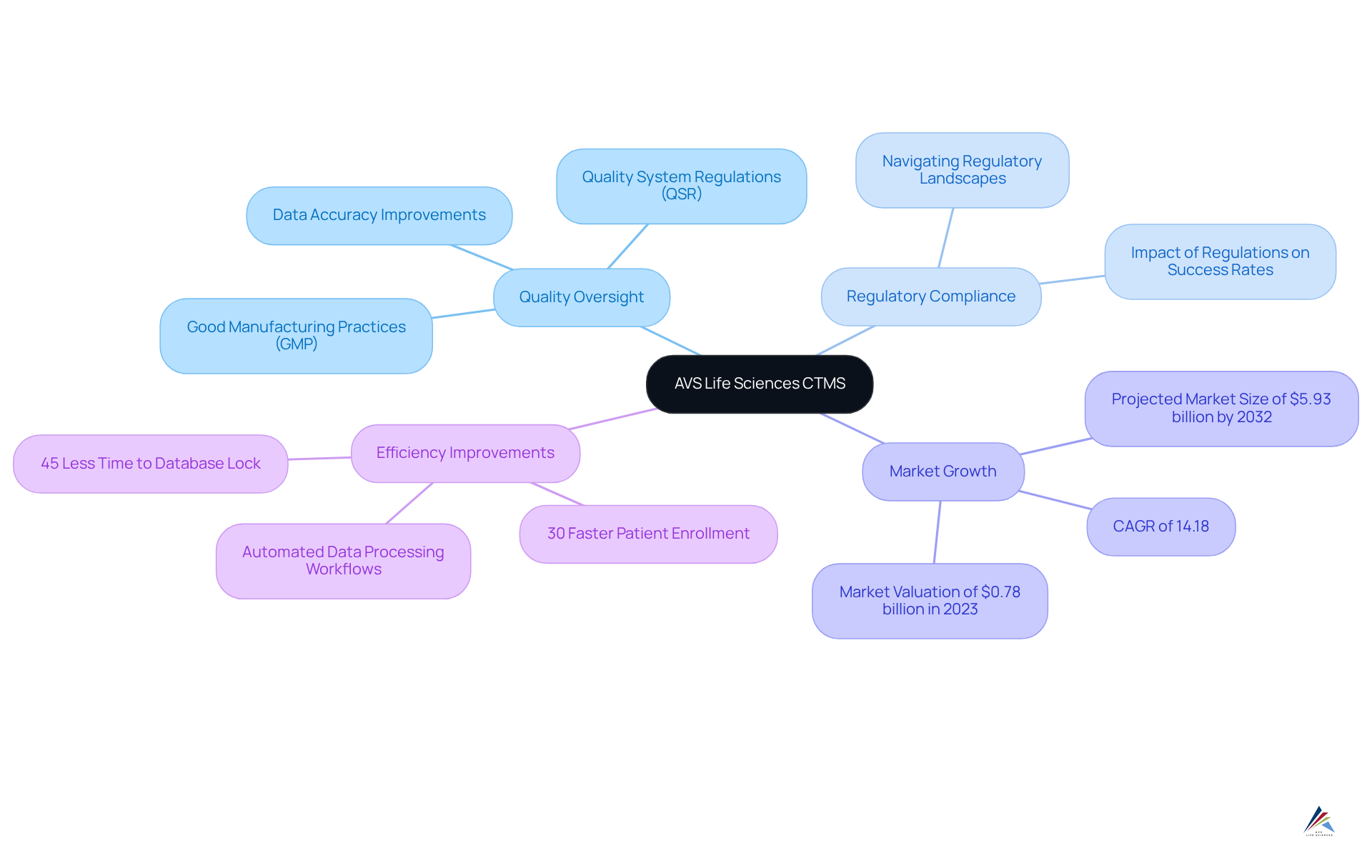
OnCore CTMS: Streamlined Management for Clinical Trials
OnCore CTMS is recognized as a premier clinical trial management software for managing research studies, providing an extensive array of features designed to simplify the entire research process. Key functionalities encompass:
- Protocol management
- Subject tracking
- Financial oversight
All aimed at enhancing operational efficiency. The system's user-friendly interface caters to both seasoned researchers and newcomers to medical studies, facilitating straightforward navigation through its diverse features.
A significant advantage of OnCore is its seamless integration with other systems, which promotes efficient data flow and fosters collaboration among research teams. This integration is crucial in an environment where the number of medical studies has surged, with over 419,632 investigations registered globally as of mid-2022, 77% of which were interventional studies. Such growth underscores the necessity for robust organizational systems like OnCore, capable of adapting to the increasing complexities of research.
Looking ahead to 2025, OnCore is poised to sustain its upward trajectory in the research oversight market, projected to reach USD 930.81 million in the U.S. alone, driven by rising research activity. The system's current market share reflects its widespread adoption, particularly among institutions focused on non-cancer research, where it has become an indispensable tool for managing studies with billable services.
Real-world applications of OnCore illustrate its effectiveness; for example, the University of Arizona Cancer Center has relied on OnCore since 2007 to uphold its National Cancer Institute designation, demonstrating its reliability in high-stakes environments. Importantly, OnCore is mandated for all prospective non-cancer-related human subject research studies with billable services, highlighting its critical role in ensuring compliance and operational efficiency. Furthermore, the system enhances research oversight by providing automated alerts and dashboards that deliver real-time information on study progress and participant monitoring, ensuring adherence and operational excellence.
In summary, the clinical trial management software OnCore CTMS not only streamlines the management of clinical studies but also empowers organizations to adapt to the evolving demands of the pharmaceutical research landscape, establishing it as an essential tool for effective research management.
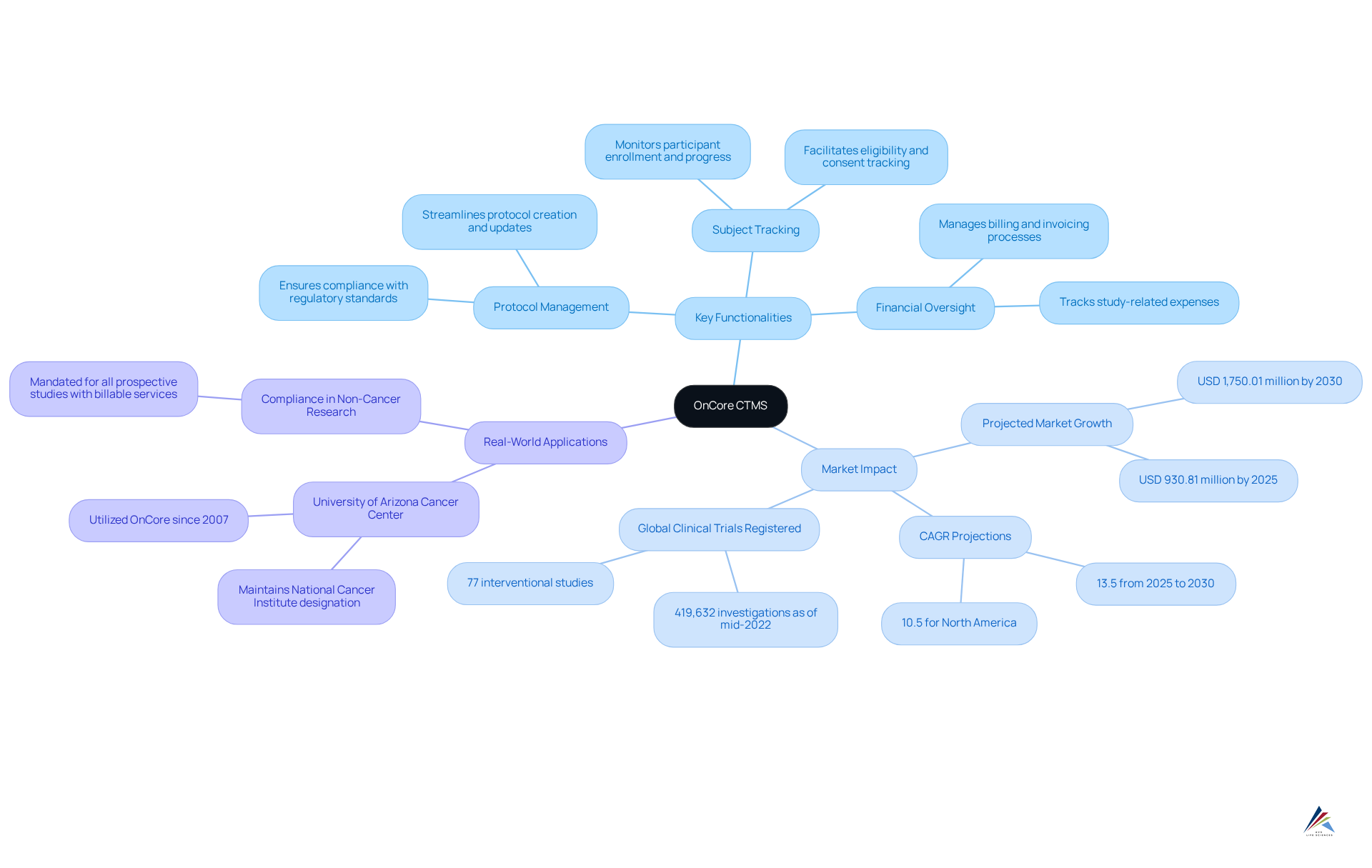
RealTime CTMS: Enhanced User Experience and Operational Efficiency
RealTime CTMS stands out in the market by prioritizing user experience, offering an intuitive platform that significantly enhances operational efficiency. This clinical trial management software includes a comprehensive suite of tools designed for managing study information, such as:
- Budgeting
- Patient tracking
- Extensive reporting capabilities
Its user-centric design notably reduces training time and minimizes the potential for errors, thereby facilitating a smoother onboarding process for research teams. Furthermore, RealTime CTMS provides immediate access to information, empowering teams to make quick, informed decisions that positively impact study outcomes. This capability is increasingly critical as the complexity of medical studies escalates, evidenced by:
- The average number of eligibility criteria per study rising
- The number of endpoints in protocols increasing by 86%
The Clinical Trial Management System Market was valued at USD 1.80 Billion in 2023, with projections indicating growth to USD 5.93 Billion by 2032, reflecting a CAGR of 14.18% from 2024 to 2032. North America led the CTMS market with a 51% revenue share in 2023, underscoring the significance of RealTime CTMS within this expanding landscape. By optimizing information organization and ensuring compliance with regulatory standards, clinical trial management software such as RealTime CTMS emerges as an essential solution in the evolving realm of research studies.
Medidata Solutions CTMS: Cloud-Based Integration for Modern Trials
Medidata Solutions CTMS provides a comprehensive clinical trial management software platform that seamlessly integrates study design and information analysis, revolutionizing the management of research studies. This innovative system fosters real-time collaboration among stakeholders, guaranteeing that all participants have immediate access to the most current information. With its advanced analytical capabilities, Medidata empowers organizations to extract valuable insights from experimental data, thereby enhancing decision-making processes and boosting efficiency. The robust cloud infrastructure not only fortifies information security and compliance but also positions Medidata as the preferred choice for leading pharmaceutical firms navigating the complexities of modern research studies.
Veeva Vault CTMS: Comprehensive Data Management for Compliance
Veeva Vault CTMS stands out as a formidable clinical trial management software for efficiently managing clinical trial information, ensuring secure storage and effortless accessibility of crucial details. Its key features encompass:
- Document management
- Compliance tracking
- Real-time reporting
All meticulously designed to meet regulatory requirements. Organizations have reported enhanced information accuracy and consistency due to stringent entry protocols, underscoring the platform's effectiveness in bolstering compliance and information integrity.
Furthermore, the platform's seamless integration with other Veeva applications amplifies its capabilities, enabling organizations to streamline research processes while upholding high standards of data integrity and compliance. This capability is particularly vital as organizations navigate the complexities of regulatory frameworks, where maintaining accurate and compliant documentation is essential for success.
The efficiency of Veeva Vault CTMS, a clinical trial management software, in ensuring adherence is underscored by numerous success stories, including case studies that illustrate the advantages of agile methodologies in research software development. As research studies progress, the significance of a thorough document management system cannot be overstated; it plays a crucial role in ensuring that all study-related documents are managed efficiently, thus aiding organizations in complying with stringent regulatory standards.
Oracle Siebel CTMS: Advanced Analytics and Reporting Features
Oracle Siebel CTMS stands out due to its sophisticated analytics and reporting capabilities, which are vital for clinical research teams aiming to make informed, data-driven decisions. The system provides customizable dashboards and real-time reporting tools, enabling users to effectively monitor key performance indicators and compliance metrics. This level of insight is essential for the early detection of potential issues, ensuring that projects stay on schedule and within budget. Furthermore, Oracle Siebel's robust information management capabilities enhance regulatory compliance, positioning it as a dependable choice for organizations navigating the complexities of the life sciences sector.
As the pharmaceutical predictive analytics market is projected to grow at a CAGR of 24.1%, reaching $11.2 billion by 2030, the integration of advanced analytics in research management is increasingly crucial. Companies leveraging these capabilities can expect improved decision-making processes. Studies indicate that organizations with fully integrated data systems experience 29% faster decision-making across healthcare and commercial operations, as reported in Accenture's Healthcare Analytics Benchmark Survey. This trend underscores the importance of utilizing advanced clinical trial management software, such as Oracle Siebel CTMS, to optimize research outcomes.
Moreover, AstraZeneca's implementation of a unified analytics platform led to a 30% increase in candidate molecules advancing to medical studies and a 25% reduction in early-stage development costs, showcasing the tangible benefits of sophisticated analytics in clinical evaluations. Addressing challenges such as managing significant costs and ensuring regulatory compliance is imperative, and the clinical trial management software provided by Oracle Siebel CTMS equips organizations with the essential tools to navigate these complexities effectively.
Castor EDC: Intuitive Electronic Data Capture for Trials
Castor EDC stands out as an intuitive electronic information capture system that streamlines the data collection process in clinical studies. Its user-friendly interface empowers researchers to create and manage electronic case report forms (eCRFs) effortlessly, leading to significant improvements in entry efficiency and a reduction in potential errors.
With real-time information integration capabilities, Castor EDC guarantees that study information remains current and readily accessible, which is crucial for maintaining information integrity and compliance. This system is essential for research teams striving to enhance their information handling procedures, as evidenced by a remarkable 40% increase in patient retention rates in studies utilizing Castor.
Furthermore, the platform's design fosters seamless collaboration among research teams, facilitating prompt feedback and modifications that are vital in today's fast-paced research landscape. Notably, as the volume of research study information escalates every two years, the demand for effective clinical trial management software has never been more critical.
Additionally, 98% of customers express satisfaction with Castor, highlighting its effectiveness and user-friendly design.
ClinCapture: Flexible and Customizable CTMS Solutions
ClinCapture presents highly adaptable and customizable Trial Management System (CTMS) solutions tailored to meet the unique requirements of research teams. The platform empowers users to modify workflows, data collection forms, and reporting tools, ensuring alignment with specific project needs. This adaptability not only enhances operational efficiency but also enables organizations to respond swiftly to evolving regulatory demands by utilizing clinical trial management software. ClinCapture's commitment to user satisfaction is reflected in its intuitive interface and comprehensive support services, solidifying its status as a leading choice for those in search of a versatile CTMS solution.
With the CTMS market projected to grow from USD 2.30 billion in 2022 to USD 4.97 billion by 2030, the importance of adaptability in study management is more critical than ever. Scott Weidley, CEO of ClinCapture, emphasizes this necessity, stating, "Our technology can accelerate remote studies and potentially have a direct effect on the speed of delivering new treatments to the public with the ultimate aim of saving lives."
Furthermore, ClinCapture's recent launch of Virtual Data Capture® (VDC®) underscores its proactive approach to managing decentralized and remote studies, addressing the increasing complexity of medical research.
Tip: When selecting a CTMS, evaluate the platform's ability to adapt to your specific study requirements and regulatory changes to ensure effective oversight and compliance.
TrialMaster: User-Friendly Design for Efficient Trial Management
TrialMaster is meticulously crafted with user experience at its core, providing a platform that streamlines the oversight of research studies. Its intuitive interface empowers users to navigate various functionalities with ease, significantly diminishing the learning curve for newcomers. Essential features such as protocol oversight, subject tracking, and comprehensive reporting tools are strategically designed to boost operational efficiency.
By prioritizing usability, TrialMaster equips research teams to manage their studies effectively while ensuring compliance with regulatory standards. Notably, user-friendly design in research study systems can enhance efficiency by up to 30%, establishing it as a crucial factor for success in the evolving landscape of medical studies.
As Forrester indicates, every $1 invested in UX can yield a remarkable return of $100, highlighting the financial advantages of emphasizing usability. As the industry progresses toward 2025, the focus on usability will intensify, with organizations increasingly acknowledging that a well-designed system not only elevates productivity but also improves user satisfaction and retention.
A relevant case study on Patient Recruitment and Retention illustrates how implementing a CTMS like TrialMaster can significantly enhance engagement and communication with participants, further validating the importance of usability in research management.
eClinicalWorks: Comprehensive Tools for Collaboration in Trials
eClinicalWorks provides a robust suite of tools designed to enhance collaboration among research stakeholders. By enabling real-time communication, document sharing, and seamless data integration, the platform guarantees that all team members have immediate access to essential information. This functionality not only enhances operational efficiency but also is pivotal in ensuring compliance with regulatory standards. As organizations increasingly recognize the importance of effective communication in clinical studies, eClinicalWorks stands out as a clinical trial management software with its user-friendly interface and comprehensive support services, making it an invaluable asset for organizations conducting clinical research.
Looking ahead to 2025, the focus on real-time communication is anticipated to intensify, with 91% of sponsors intending to utilize remote monitoring tools, underscoring the trend towards technological adoption. Yet, challenges persist; 48% of sites express concerns regarding the affordability of new technology, while 40% report that integration issues impede their technology adoption. Importantly, successful implementations of eClinicalWorks have showcased notable enhancements in trial efficiency, illustrating the platform's effectiveness in promoting collaboration and improving overall project execution. This combination of features and proven results positions eClinicalWorks as a leader in the industry, ready to meet the evolving demands of clinical studies.
Conclusion
The landscape of clinical trial management software is evolving rapidly, with innovative solutions designed to meet the increasing demands of the pharmaceutical and biotechnology sectors. Each software solution highlighted offers unique features and capabilities that streamline the research process, enhance operational efficiency, and ensure regulatory compliance. As organizations strive for excellence in clinical trials, selecting the right CTMS becomes crucial for success.
Key insights from the discussion reveal that comprehensive tools like AVS Life Sciences, OnCore, and RealTime CTMS not only facilitate effective management of clinical studies but also significantly improve data accuracy and study effectiveness. The emphasis on user experience, as seen with platforms like TrialMaster and eClinicalWorks, showcases the importance of intuitive design in promoting productivity and satisfaction among research teams. Furthermore, the integration of advanced analytics and cloud-based solutions, such as those offered by Oracle Siebel and Medidata, empowers organizations to make data-driven decisions and foster collaboration among stakeholders.
As the clinical trial management software market continues to expand, organizations must prioritize adaptability and user-centric design when selecting their systems. Embracing these innovative solutions will not only enhance the efficiency of clinical trials but also contribute to the successful delivery of new treatments to the public. The future of clinical research depends on leveraging technology to navigate the complexities of regulatory requirements and study management, ultimately leading to improved patient outcomes and advancements in healthcare.
Frequently Asked Questions
What is AVS Life Sciences CTMS?
AVS Life Sciences CTMS is a comprehensive clinical trial management software that integrates quality oversight and regulatory compliance solutions specifically designed for the pharmaceutical and biotechnology sectors.
How does AVS Life Sciences CTMS ensure compliance?
It ensures compliance by adhering to Good Manufacturing Practices (GMP) and Quality System Regulations (QSR), helping organizations navigate complex regulatory landscapes.
What are the benefits of using AVS Life Sciences CTMS?
The benefits include enhanced operational efficiency, improved success rates in research studies, and essential tools for managing the product lifecycle effectively.
What is the projected market growth for clinical trial management software?
The market for clinical trial management software is projected to grow at a CAGR of 14.18% from 2024 to 2032, driven by the increasing demand for innovative pharmaceuticals.
What features does OnCore CTMS offer?
OnCore CTMS offers features such as protocol management, subject tracking, and financial oversight, all designed to streamline the research process.
How does OnCore CTMS support research teams?
OnCore CTMS supports research teams by providing a user-friendly interface that facilitates easy navigation and seamless integration with other systems for efficient data flow.
What is the significance of OnCore CTMS in the research oversight market?
OnCore CTMS is significant as it is mandated for all prospective non-cancer-related human subject research studies with billable services, ensuring compliance and operational efficiency.
What advantages does RealTime CTMS provide?
RealTime CTMS offers an intuitive platform that enhances user experience, reduces training time, minimizes errors, and provides immediate access to study information for informed decision-making.
What tools are included in RealTime CTMS?
RealTime CTMS includes tools for budgeting, patient tracking, and extensive reporting capabilities.
What is the market value and growth projection for RealTime CTMS?
The Clinical Trial Management System Market was valued at USD 1.80 billion in 2023, with projections indicating growth to USD 5.93 billion by 2032, reflecting a CAGR of 14.18% from 2024 to 2032.
