10 Essential CAPA Examples for Pharmaceutical Compliance Officers
Overview
The article presents essential examples of Corrective and Preventive Actions (CAPA) that pharmaceutical compliance officers can employ to enhance regulatory compliance and product quality. It underscores the critical nature of systematic approaches, including:
- Thorough documentation
- Root cause analysis
- Continuous improvement
These elements are vital for effectively addressing non-conformities and preventing future issues within the pharmaceutical industry. By implementing these strategies, compliance officers can significantly elevate their practices, ensuring adherence to regulatory standards while fostering a culture of quality and accountability.
Introduction
The pharmaceutical industry operates under a stringent regulatory framework where compliance is non-negotiable. Corrective and Preventive Actions (CAPA) are crucial mechanisms that organizations must implement to address non-conformities and enhance product quality. This article delves into ten essential CAPA examples specifically tailored for compliance officers, offering insights into best practices and methodologies that can transform compliance efforts into a robust quality management system.
How can these CAPA examples not only prevent regulatory pitfalls but also foster a culture of continuous improvement within pharmaceutical organizations?
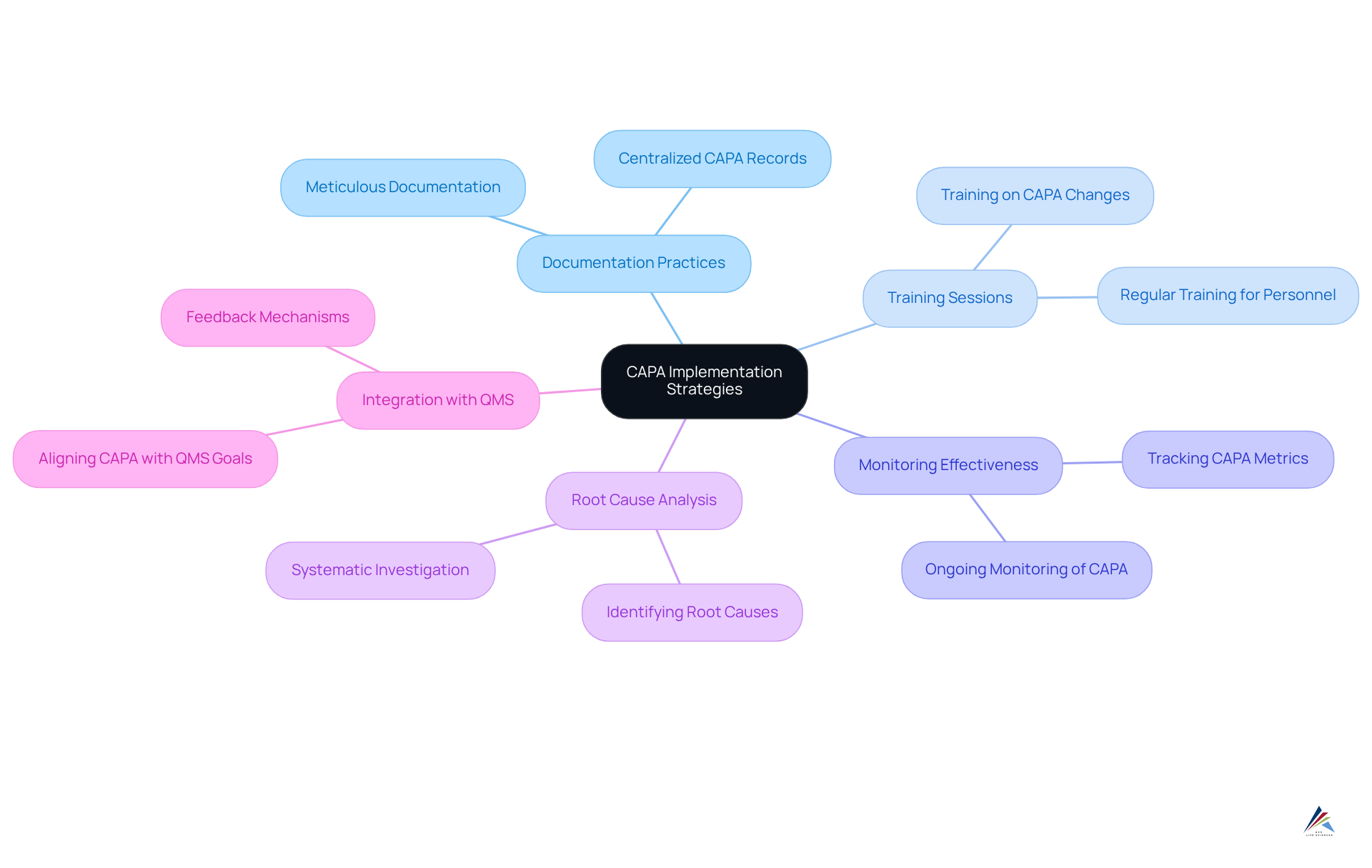
AVS Life Sciences: Comprehensive CAPA Implementation Strategies
AVS Life Sciences employs a systematic method for implementing corrective and preventive actions, placing a strong emphasis on identifying root causes of non-conformities and developing actionable plans to address them. Key strategies encompass:
- Meticulous documentation practices
- Regular training sessions for personnel
- Ongoing monitoring of CAPA effectiveness, as illustrated by a capa example
This integration not only resolves current challenges but also proactively prevents future incidents, significantly enhancing compliance and the overall management system. By fostering a culture of continuous improvement, AVS Life Sciences positions organizations to meet regulatory demands while upholding high standards of product quality.
Notably, have contributed to approximately 40% of Form 483 observations issued to pharmaceutical producers, underscoring the urgent need for efficient capa example methodologies. As Etienne Nichols articulates, "Corrective and Preventive Action refers to a systematic approach to risk management in manufacturing..."
This unwavering commitment to excellence is further evidenced by AVS Life Sciences' impressive 80% repeat business rate, showcasing their effectiveness in managing corrective and preventive actions.
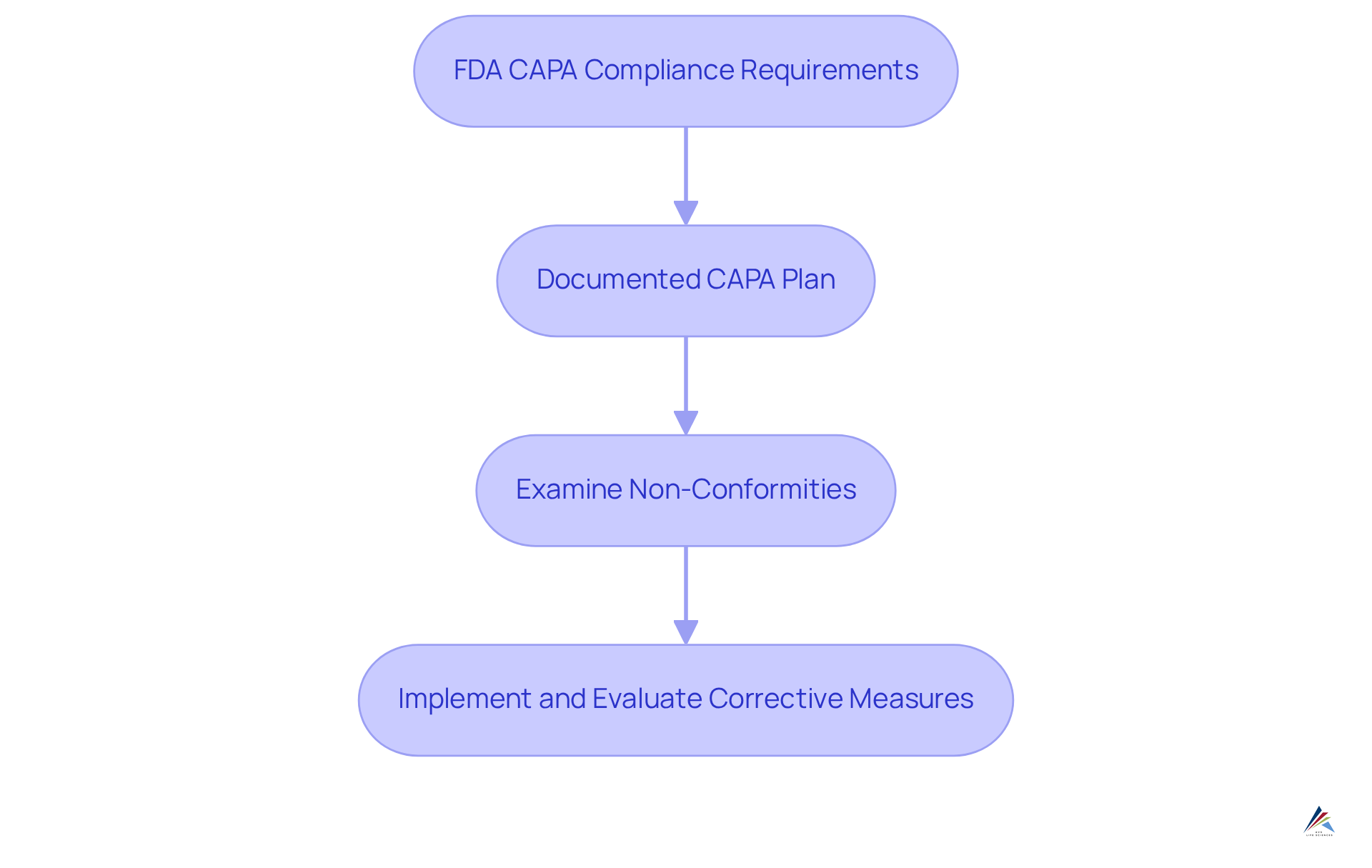
FDA Guidelines: Key CAPA Requirements for Compliance
The FDA mandates that corrective and preventive actions be meticulously documented and utilize a systematic approach for identifying, investigating, and resolving issues pertaining to standards. Key criteria include:
- The establishment of a documented corrective and preventive action plan
- Immediate examination of non-conformities
- The implementation of corrective measures that are evaluated for effectiveness
Compliance officers must ensure their corrective and preventive action systems are in strict alignment with these guidelines to avert regulatory penalties and uphold product quality.
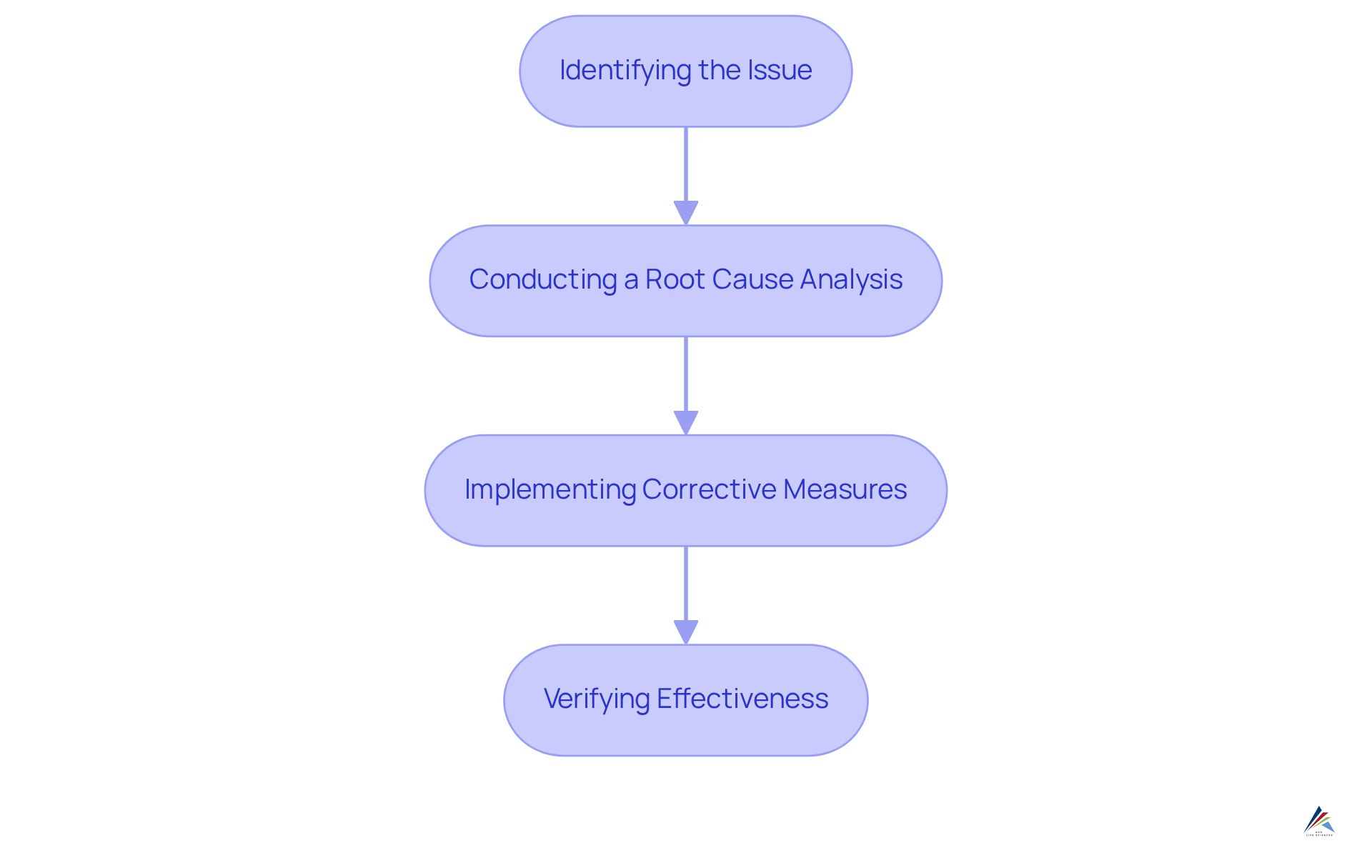
FoodDocs: CAPA Process Steps in Food Safety Management
FoodDocs outlines a corrective action plan that encompasses several critical steps:
- Identifying the issue
- Conducting a root cause analysis
- Implementing corrective measures
- Verifying the effectiveness of those measures
For pharmaceutical regulatory officers, embracing a similarly organized approach can significantly enhance their corrective and preventive action processes. This ensures that all potential risks are addressed systematically and effectively, reinforcing the importance of compliance in safeguarding public health.
PMC: Enhancing Pharmaceutical Quality with CAPA Framework
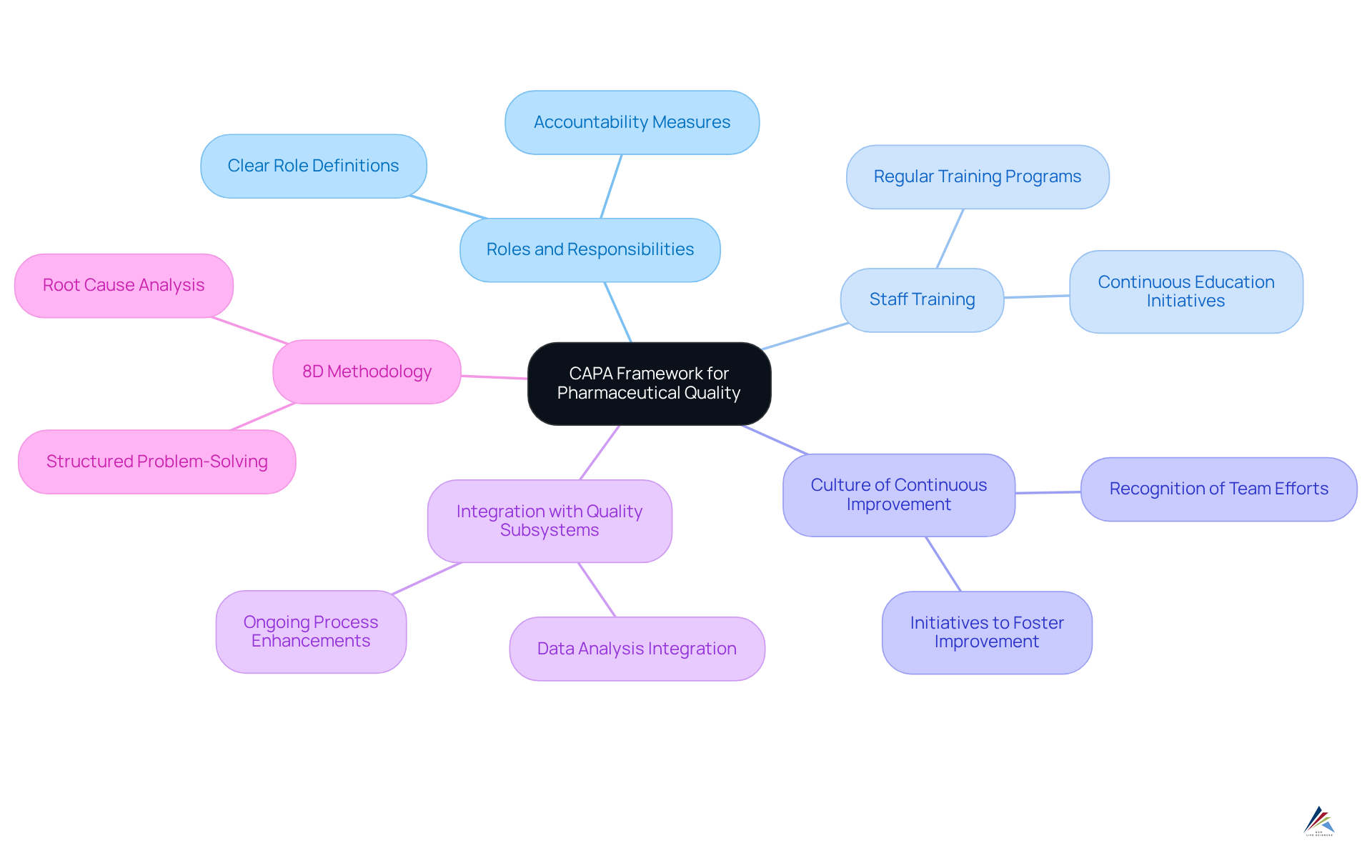
The Pharmaceutical Manufacturing Consortium (PMC) emphasizes that a clearly outlined is essential for enhancing pharmaceutical standards. This framework must:
- Delineate clear roles and responsibilities
- Provide regular training for staff
- Foster a culture of continuous improvement
By cultivating an environment that prioritizes excellence, compliance officers can ensure their organizations not only meet regulatory requirements but also exceed industry standards. Furthermore, integrating the corrective and preventive action system with other quality subsystems is crucial for its effectiveness. The 8D methodology serves as a systematic problem-solving technique to bolster corrective and preventive action efforts, guaranteeing thorough issue resolution. Given that the biologics market is projected to grow at a compound annual growth rate of 15% until 2027, the importance of maintaining high standards through effective corrective and preventive action execution cannot be overstated. As Kanaka P. Kannaiah asserts, 'The corrective and preventive action system is an indispensable component of quality assurance in the pharmaceutical industry, essential for maintaining high standards of drug manufacturing to ensure quality and regulatory compliance.
Qualio: CAPA Requirements for Medical Device Compliance
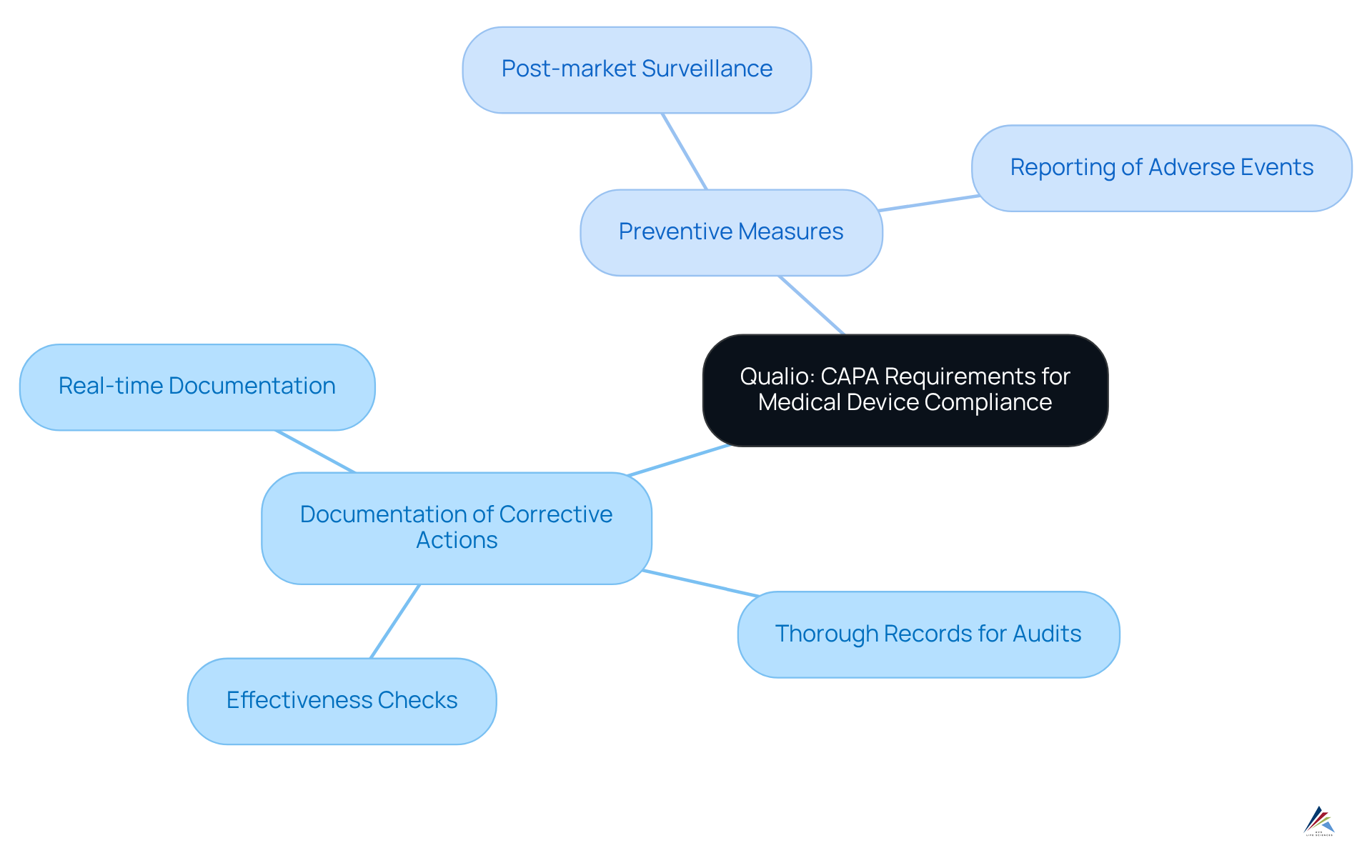
Qualio elucidates the critical requirements for medical devices, emphasizing the necessity for comprehensive documentation of all corrective actions implemented. This includes the establishment of aimed at mitigating future risks. Compliance officers must ensure that their corrective and preventive action systems are sufficiently robust to tackle the unique challenges posed by medical device regulations. These challenges encompass:
- Post-market surveillance
- Reporting of adverse events
These are essential for maintaining regulatory compliance and ensuring patient safety.
Advisera: Distinguishing Corrective and Preventive Actions in CAPA
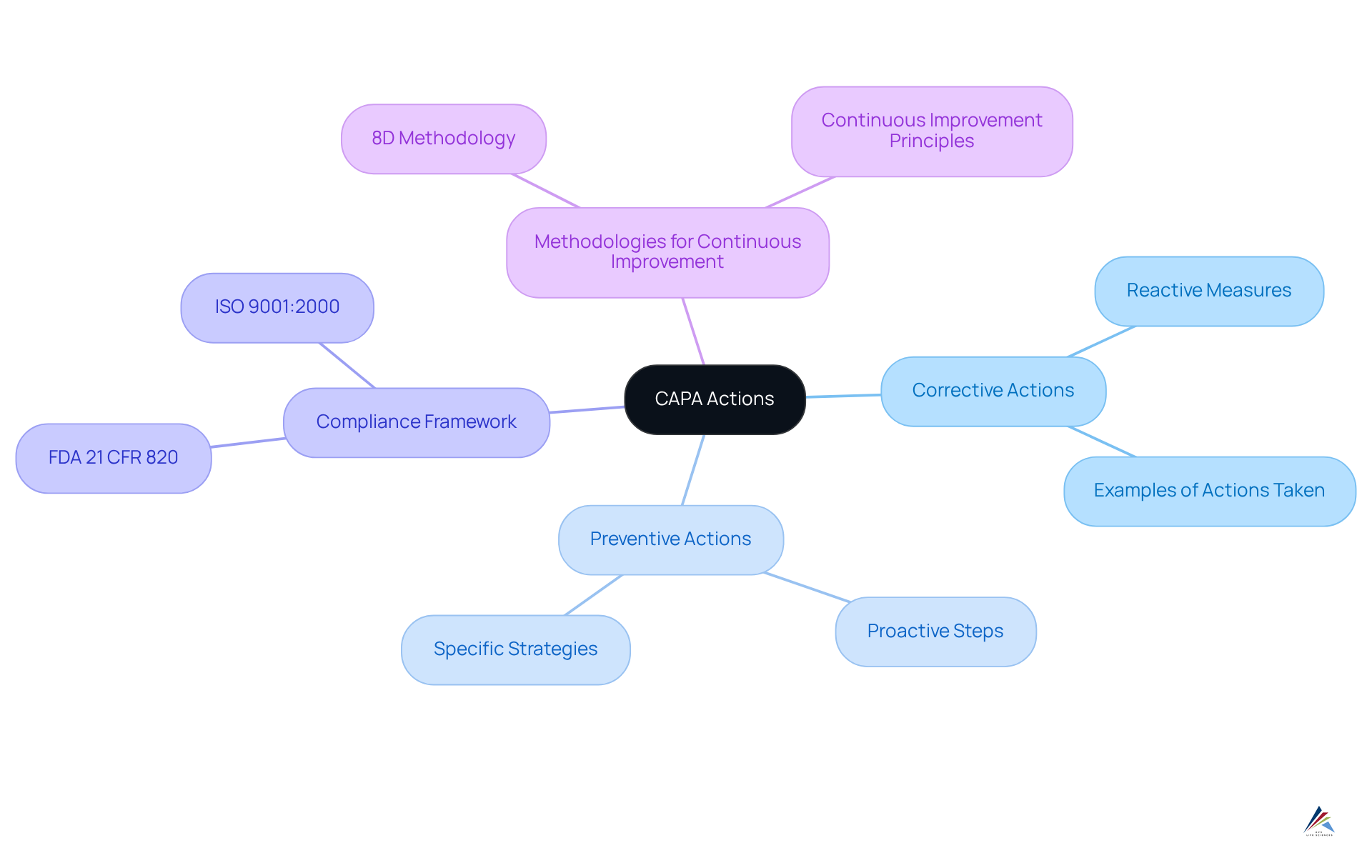
Advisera emphasizes that corrective actions are reactive measures taken to address specific non-conformities, while preventive actions are proactive steps aimed at preventing potential issues from arising. Compliance officers must ensure that their processes include a CAPA example that encompasses both categories of actions, fostering a comprehensive management approach that not only addresses current issues but also mitigates future risks.
It is essential to document these procedures in accordance with FDA 21 CFR 820, which provides a CAPA example that mandates corrective and preventive actions in medical device manufacturing. Utilizing the can provide a structured framework for effectively integrating these actions.
Moreover, adopting continuous improvement principles as specified in ISO 9001:2000 can significantly enhance the overall management system. By applying best practices that blend corrective and preventive measures, organizations can greatly improve adherence and maintain high standards of product quality and safety.
Greenlight Guru: Mastering the CAPA Process for Compliance
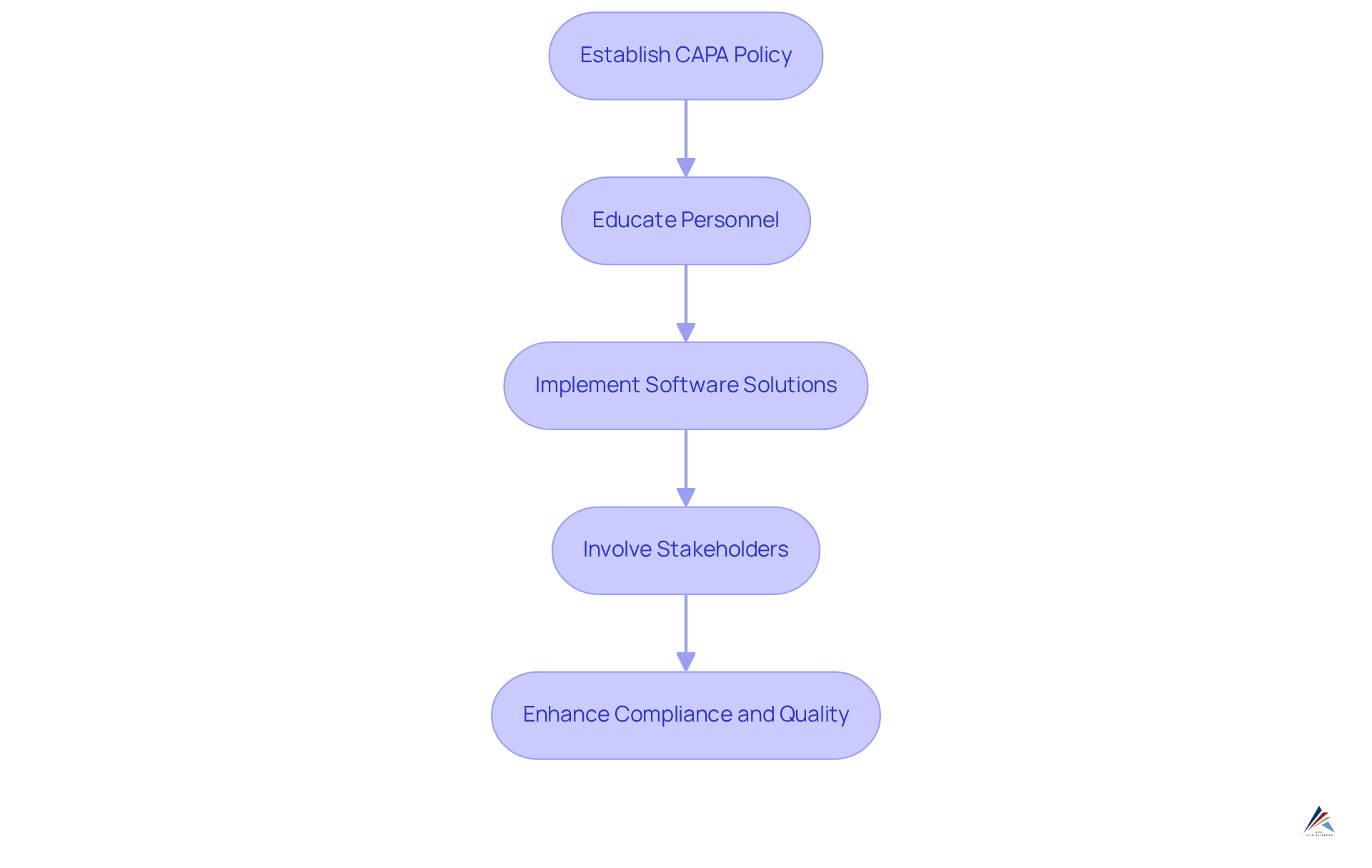
Mastering the corrective and preventive action process is essential for compliance officers in the pharmaceutical industry, involving several critical practices. Establishing a clear corrective and preventive action policy is paramount. This policy should outline the procedures for identifying, documenting, and , ensuring that all team members understand their roles and responsibilities. Effective corrective and preventive action policies not only adhere to regulatory requirements but also promote a culture of continuous enhancement within organizations.
Educating personnel on corrective and preventive action methods greatly influences operational efficiency. Regular training sessions equip employees with the necessary skills to conduct thorough root cause analyses and implement corrective actions effectively. Studies indicate that organizations investing in employee education see substantial returns, enhancing both engagement and knowledge retention, with a reported ROI of $2.29 for every dollar spent.
Employing software applications for corrective and preventive action documentation enhances overall workflow. Sophisticated corrective and preventive action management systems automate documentation, tracking, and reporting, enabling compliance officers to concentrate on strategic oversight instead of administrative duties. However, challenges associated with independent corrective and preventive action software, such as inflexibility and lack of connectivity between quality processes, should be considered when selecting tools. These systems facilitate real-time data access, enabling organizations to identify bottlenecks and ensure timely corrective actions.
The capa example of effective corrective and preventive action policies in pharmaceutical companies demonstrates the value of these practices. Firms that emphasize clear guidelines, thorough training, and strong software solutions frequently report enhanced adherence rates and fewer occurrences of regulatory citations. Additionally, involving stakeholders in the corrective and preventive action framework improves results through teamwork and collective accountability. By creating a robust corrective and preventive action framework, compliance officers can enhance their organizations' capability to manage intricate regulatory environments while upholding high standards of quality and safety.
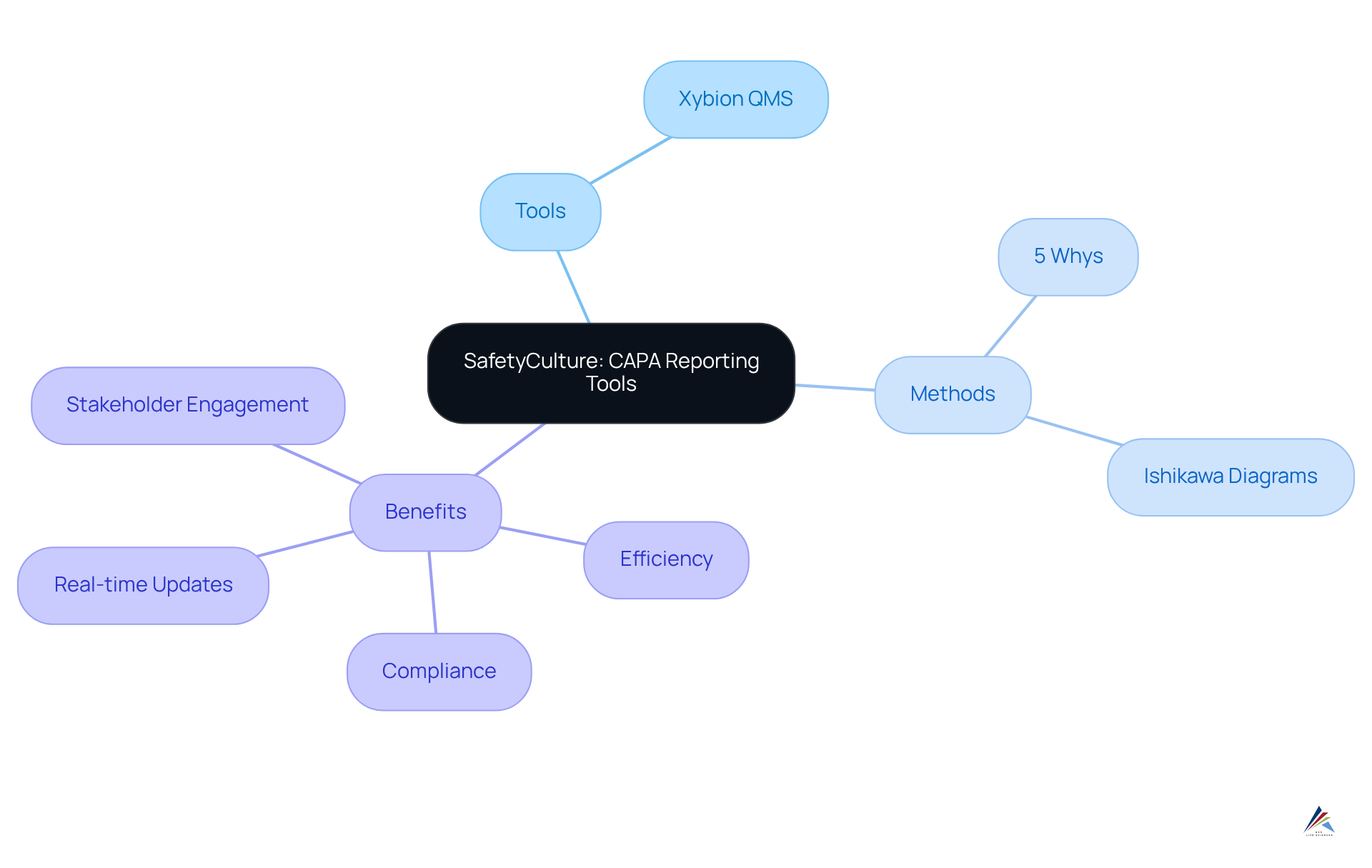
SafetyCulture: Effective CAPA Reporting Tools and Templates
SafetyCulture emphasizes the necessity of employing efficient reporting tools and templates, such as Xybion QMS, to streamline the documentation workflow. These tools facilitate straightforward , allow for real-time updates, and ensure that all relevant stakeholders remain informed. Compliance officers must invest in these tools to enhance their corrective and preventive action processes, thereby increasing efficiency and adherence to regulatory standards.
Moreover, the application of root cause analysis methods—like the '5 Whys' technique and Ishikawa diagrams—is essential for identifying underlying issues and fostering continuous improvement in corrective and preventive action management. By leveraging advanced documentation tools and methodologies, organizations can swiftly address issues while upholding high-quality standards.
WCG Clinical: CAPA Principles in Clinical Research Compliance
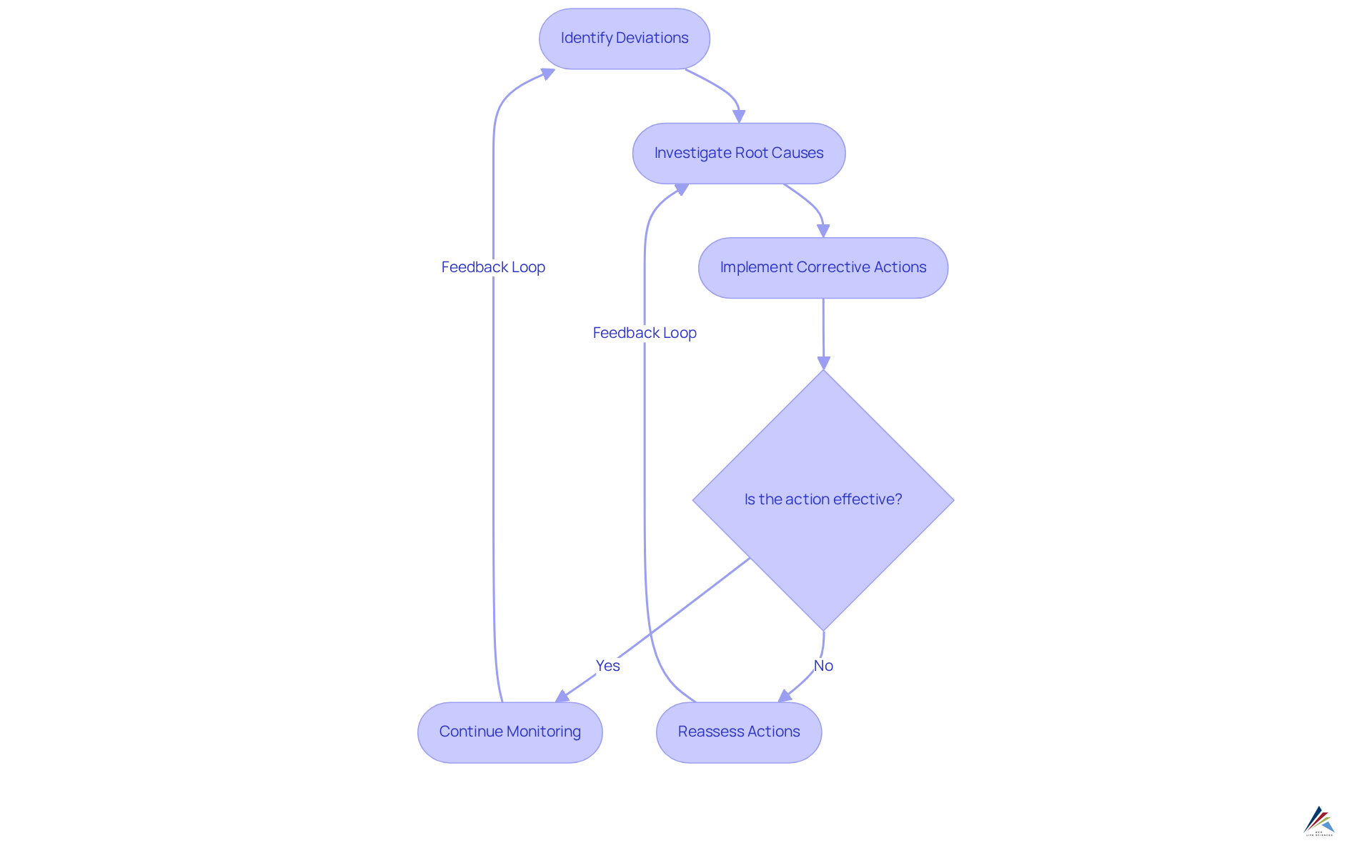
Effective corrective and preventive action principles in clinical research are essential for maintaining compliance and ensuring the integrity of clinical trials. These principles involve systematically identifying deviations from established study protocols, thoroughly investigating their root causes, and implementing to prevent future occurrences. Compliance officers must adjust their corrective and preventive action processes to tackle the distinct challenges presented by clinical research, including the intricacies of regulatory demands and the need for prompt reporting.
Recent difficulties, such as operational issues resulting from the COVID-19 pandemic, have underscored the significance of prompt identification and resolution of problems to improve the effectiveness of corrective and preventive actions. Delays in reporting deviations can lead to compounded issues, affecting trial integrity and participant safety. Expert insights suggest that fostering a culture of transparency and proactive communication within research teams can significantly improve the identification of deviations. As noted by Francesca Fiorentino, 'Compliance is defined as the extent to which the patient's behavior matches the prescriber's recommendations,' emphasizing the critical nature of adherence in clinical trials.
By utilizing strong corrective and preventive action strategies, organizations can not only meet regulatory standards but also improve the overall quality and reliability of their clinical research outcomes. Furthermore, it is crucial to recognize that only 13% of studies have utilized methods to handle compliance as described in their protocols, underscoring the need for improved practices in this area.
Software Testing Help: Overview of CAPA Processes and Best Practices
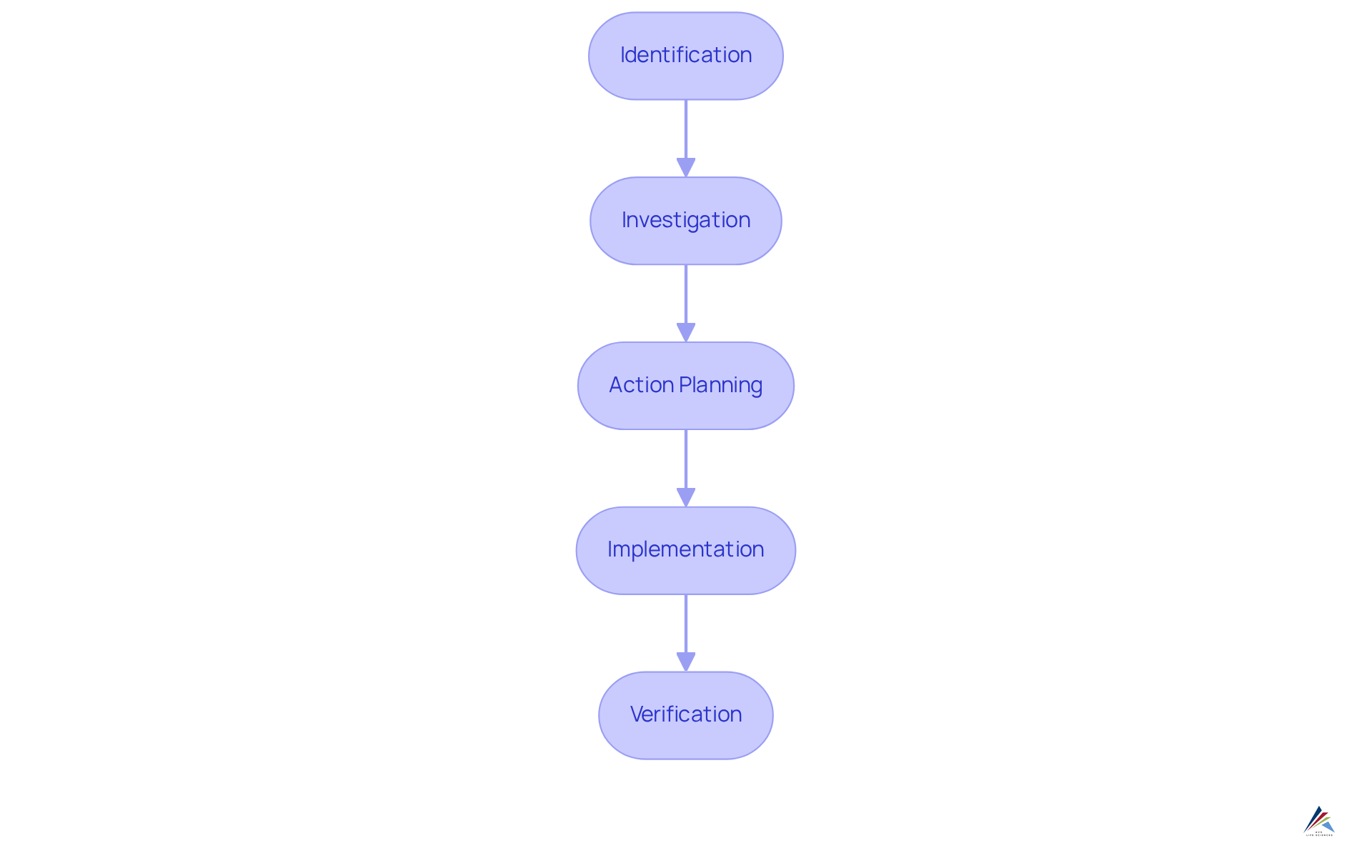
The corrective and preventive action framework serves as a systematic method encompassing several essential stages: identification, investigation, action planning, implementation, and verification. Effective management of corrective and preventive actions underscores the necessity of clear documentation and the active participation of cross-functional teams. These teams, composed of individuals from diverse departments, enhance the system by offering varied perspectives and expertise, which are vital for and effective solutions.
Recent trends indicate that organizations leveraging cross-functional collaboration in their corrective and preventive action processes achieve improved effectiveness and compliance outcomes. Notably, companies employing automated corrective and preventive actions report a higher percentage of products meeting standards, achieving adherence rates of 100% to 99% for the top 50%.
Regular evaluations of the effectiveness of corrective and preventive actions are crucial, as these measures have been the primary cause of FDA 483 inspectional observations since 2010. By implementing these best practices, regulatory officers can ensure that their corrective and preventive action processes are not only robust and effective but also aligned with industry standards, ultimately fostering a culture of continuous improvement.
As Nathan Conover aptly articulates, "CAPA is the immune system of your organization," highlighting its critical role in sustaining quality and compliance.
Conclusion
The significance of effective Corrective and Preventive Actions (CAPA) in the pharmaceutical industry is paramount. By implementing robust CAPA strategies, organizations can not only address existing compliance issues but also proactively mitigate potential risks, thereby ensuring the highest standards of product quality and regulatory adherence. This article has highlighted various essential CAPA examples and best practices that compliance officers can adopt to enhance their operational frameworks.
Key insights from the article emphasize the importance of meticulous documentation, regular training, and the integration of cross-functional teams in the CAPA process. Companies such as AVS Life Sciences and FoodDocs exemplify how systematic approaches to identifying root causes and implementing corrective measures lead to improved compliance outcomes. Moreover, aligning CAPA processes with FDA guidelines and employing advanced reporting tools is vital for maintaining regulatory compliance and fostering a culture of continuous improvement.
In summary, the pharmaceutical sector operates under constant scrutiny, making effective CAPA practices critical for success. Compliance officers are urged to adopt these strategies not only to meet regulatory requirements but also to enhance overall operational efficiency. By prioritizing comprehensive CAPA frameworks, organizations can safeguard public health, improve product quality, and ultimately thrive in an increasingly competitive landscape. Embracing these best practices is a decisive step toward achieving excellence in pharmaceutical compliance and quality assurance.
Frequently Asked Questions
What is the primary focus of AVS Life Sciences in their CAPA implementation strategies?
AVS Life Sciences focuses on identifying root causes of non-conformities and developing actionable plans to address them, emphasizing meticulous documentation, regular training, and ongoing monitoring of CAPA effectiveness.
How does AVS Life Sciences enhance compliance and product quality?
By fostering a culture of continuous improvement and implementing systematic CAPA strategies, AVS Life Sciences positions organizations to meet regulatory demands and uphold high standards of product quality.
What percentage of Form 483 observations issued to pharmaceutical producers is attributed to deficiencies in CAPA?
Approximately 40% of Form 483 observations are attributed to deficiencies in corrective and preventive actions.
What are the FDA's key requirements for CAPA compliance?
The FDA requires a documented CAPA plan, immediate examination of non-conformities, and the implementation of corrective measures that are evaluated for effectiveness.
Why is it important for compliance officers to align their CAPA systems with FDA guidelines?
It is crucial to avoid regulatory penalties and to maintain product quality by ensuring that CAPA systems are in strict alignment with FDA guidelines.
What are the critical steps in the CAPA process as outlined by FoodDocs?
The critical steps include identifying the issue, conducting a root cause analysis, implementing corrective measures, and verifying the effectiveness of those measures.
How can pharmaceutical regulatory officers benefit from a structured CAPA approach?
Embracing a structured CAPA approach can significantly enhance their processes by ensuring that all potential risks are addressed systematically and effectively, reinforcing compliance and public health safety.
