10 Essential Benefits of cGMP Supplements for Compliance and Quality

Overview
The essential benefits of cGMP supplements for compliance and quality are significant, encompassing:
- Improved product safety
- Enhanced consumer trust
- Competitive market advantages
Adherence to cGMP standards mitigates contamination risks and regulatory penalties, while fostering a culture of continuous improvement. This commitment not only leads to operational efficiencies but also strengthens brand reputation within the dietary supplement industry. By prioritizing compliance, organizations position themselves as leaders in safety and quality, ultimately driving consumer loyalty and market success.
Introduction
The dietary supplement industry is undergoing a significant transformation as the demand for quality and safety escalates. Current Good Manufacturing Practices (cGMP) serve as a pivotal framework that not only ensures compliance but also enhances product integrity and fosters consumer trust.
This article explores the ten essential benefits of cGMP supplements, highlighting how these practices can elevate operational efficiency, strengthen brand reputation, and open doors to global market opportunities.
As organizations endeavor to meet rigorous regulatory standards, the critical question arises: how can the adoption of cGMP practices propel companies forward in an increasingly competitive environment?
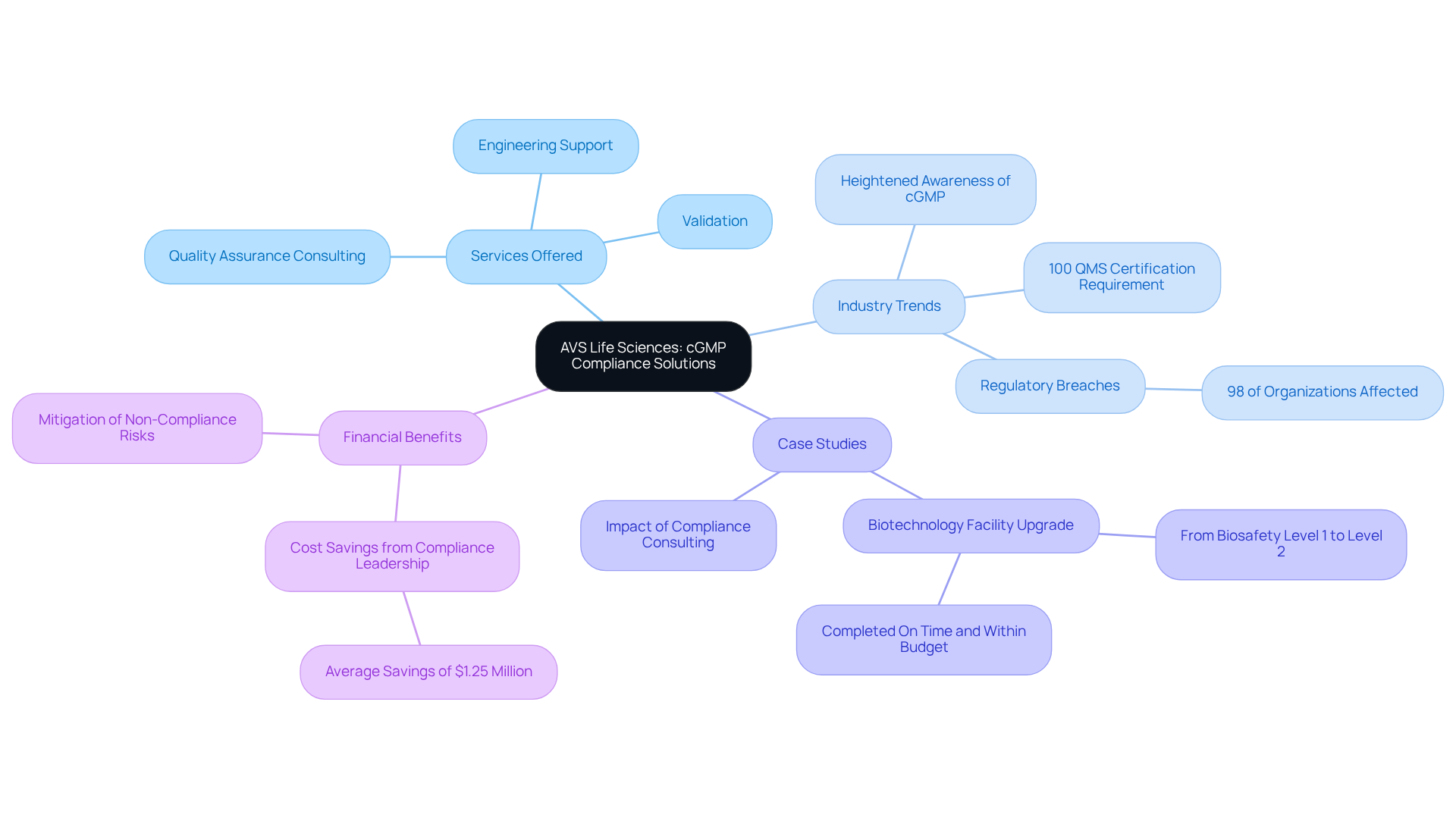
AVS Life Sciences: Comprehensive Quality Compliance Solutions for cGMP Supplements
presents a robust suite of services meticulously designed to ensure dietary supplements adhere to current Good Manufacturing Practices (cGMP). With an unwavering commitment to excellence in management and regulatory compliance, AVS tailors its solutions to empower clients in adeptly navigating the intricate landscape of life sciences. Their expertise encompasses validation, engineering support, and quality assurance consulting, establishing them as a trusted partner for organizations striving to enhance their manufacturing practices. AVS's proactive approach guarantees that clients receive personalized guidance throughout the compliance journey, including support with regulatory submissions.
Recent trends underscore a heightened awareness of the critical importance of cGMP supplements and cGMP certification within the dietary supplement industry, with 100% of contracts signed requiring Quality Management System (QMS) certification. This evolution underscores the imperative for companies to embrace stringent management practices to mitigate non-compliance risks, which can lead to substantial fines and detrimental health consequences for consumers.
The effective implementation of cGMP supplements has been demonstrated by numerous dietary supplement firms, showcasing the transformative impact of compliance consulting. For example, AVS Life Sciences facilitated a biotechnology company's upgrade of its GMP facility, advancing from a Biosafety Level 1 to a Level 2 GMP facility, completing the project punctually and within budget. This case exemplifies how effective management systems can enhance operational outcomes and ensure adherence to legal standards.
Industry leaders emphasize the pivotal role of cGMP in maintaining product standards and safeguarding consumer safety. Notably, 98% of organizations have encountered regulatory breaches in recent years, highlighting the pressing need for robust quality management frameworks. Engaging regulatory consulting services not only enhances operational efficiency but also protects against penalties and reputational harm, ultimately fostering public trust and improving health outcomes.
In conclusion, the adoption of cGMP supplements practices is vital for dietary supplement companies seeking to excel in a competitive environment. By prioritizing management standards and regulatory compliance, organizations can achieve significant cost savings while ensuring the delivery of safe, effective products to consumers. To amplify these advantages, companies are encouraged to consider appointing a dedicated compliance leader, potentially saving an average of $1.25 million in compliance costs, thereby reinforcing the financial benefits of strong management systems.
Ensures Product Quality and Safety Through cGMP Certification
Good manufacturing practices certification is essential for producers of cgmp supplements, ensuring that products are manufactured in a controlled environment, which significantly reduces the risk of contamination and errors. By adhering to current good manufacturing practices, producers can confidently guarantee the identity, strength, and purity of their offerings, especially when using cgmp supplements.
AVS Life Sciences provides specialized quality solutions, including:
to assist clients in achieving and maintaining regulatory compliance. This certification not only protects consumer health but also enhances the manufacturer's credibility in the marketplace, fostering increased trust among consumers.
Recent studies indicate that products with good manufacturing practice certification are perceived as more reliable, leading to heightened consumer confidence. Regulatory experts emphasize that maintaining stringent Good Manufacturing Practices is critical for mitigating contamination risks, especially in the cgmp supplements sector, where product integrity is paramount.
Ultimately, serves as a hallmark of quality, reinforcing the commitment to consumer safety and trust, while exemplifying the proven excellence of AVS Life Sciences in delivering successful outcomes.
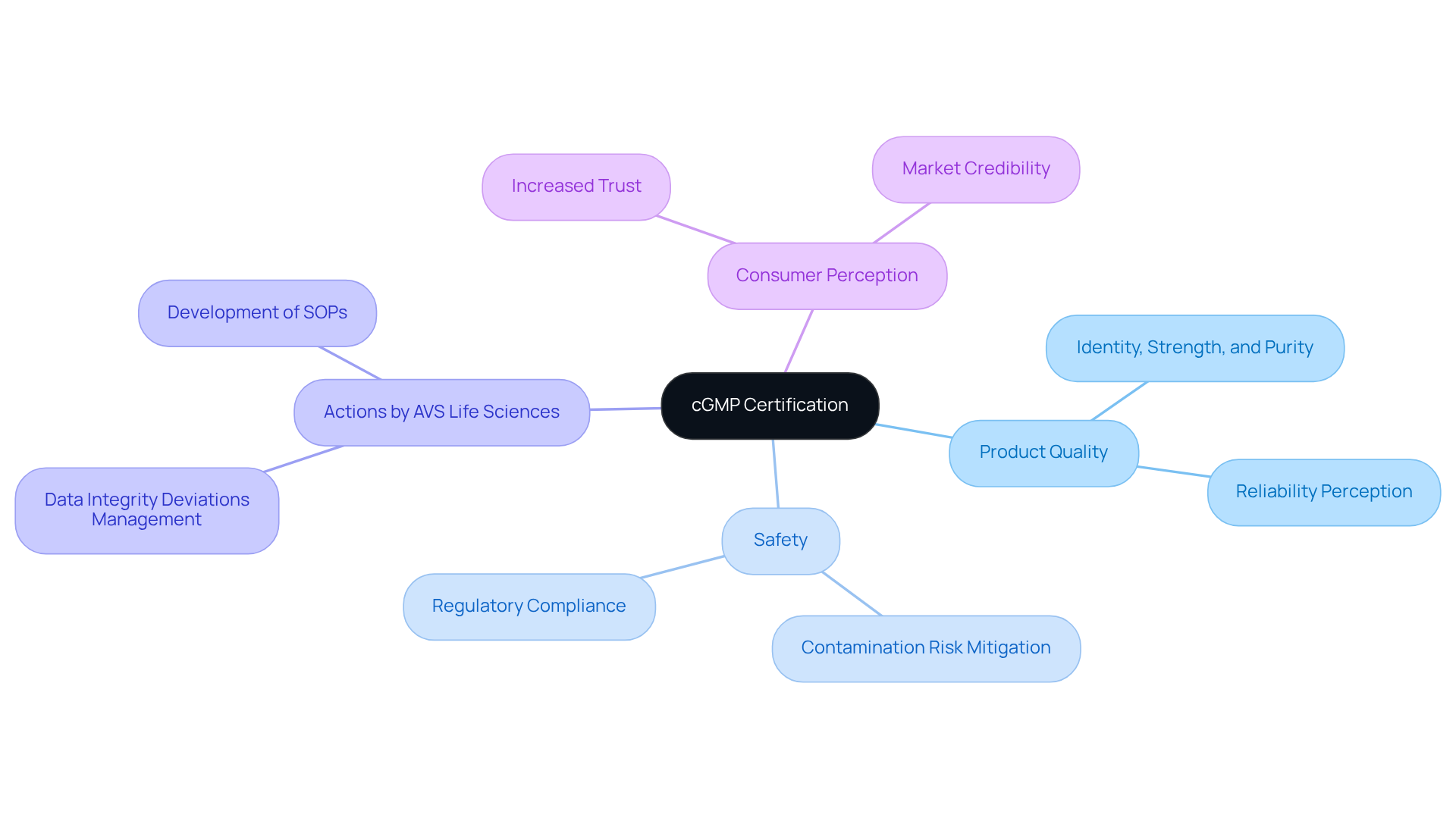
Maintains Regulatory Compliance for Dietary Supplements
Ensuring adherence to regulations stands as a cornerstone of current Good Manufacturing Practices (cGMP supplements) for dietary products. Compliance with the FDA's 21 CFR Part 111 guidelines necessitates that manufacturers of cGMP supplements uphold rigorous standards throughout the production, packaging, and labeling processes. This commitment not only safeguards consumer health but also mitigates the risk of costly penalties and product recalls.
To bolster adherence efforts, AVS Life Sciences provides designed to navigate the complexities of regulatory requirements. Additionally, common inquiries regarding good manufacturing practices are addressed in our FAQs, offering practical insights for producers.
Organizations prioritizing regulatory adherence can sidestep the pitfalls associated with regulatory scrutiny, thereby enhancing their reputation and fostering consumer trust.
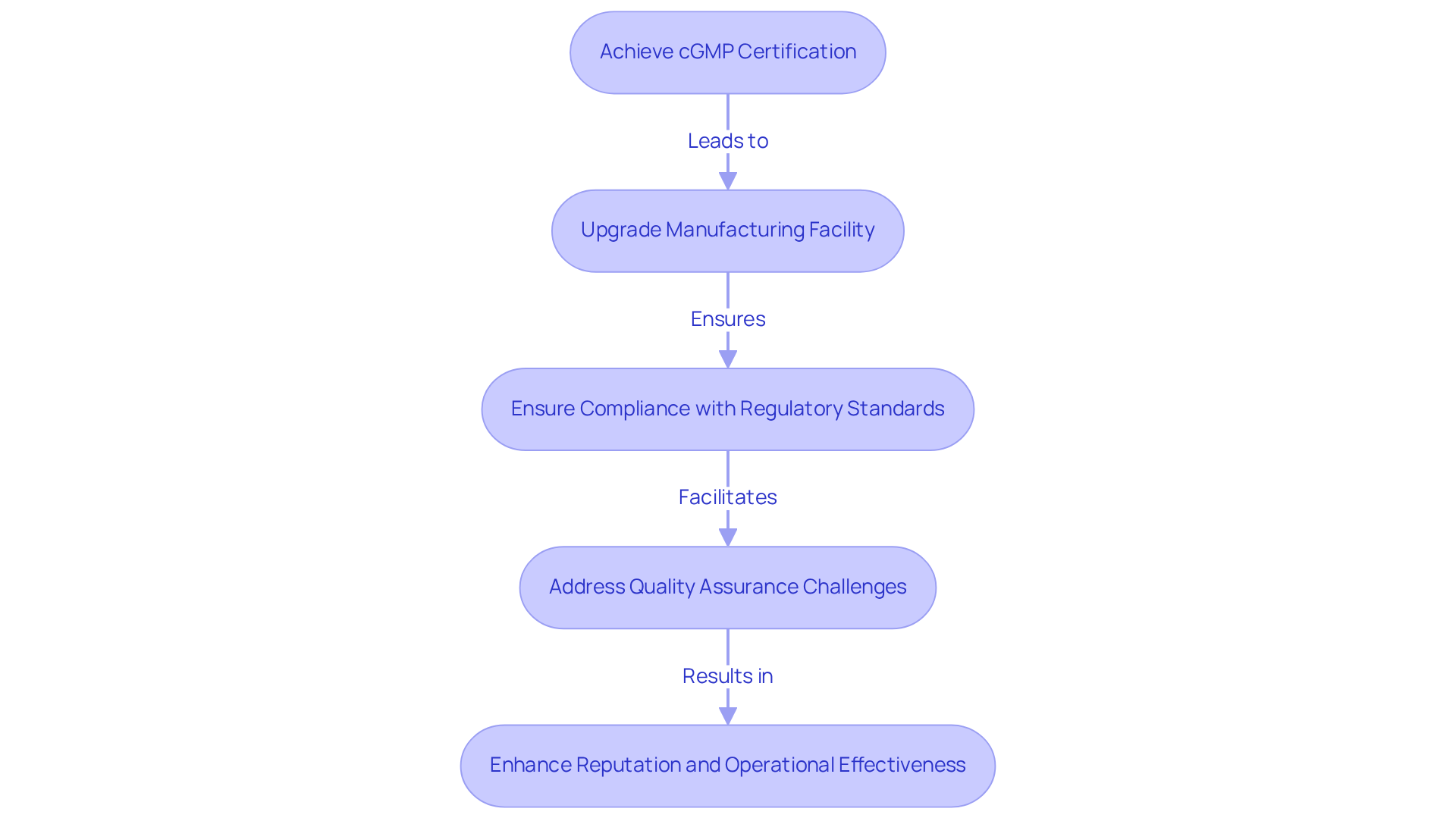
Provides Competitive Advantage in the Dietary Supplement Market
Achieving cGMP certification gives a competitive edge in the market for cGMP supplements. Brands that demonstrate adherence to [high-quality manufacturing standards](https://grandviewresearch.com/industry-analysis/dietary-supplements-market-report), such as those that produce cGMP supplements, are more likely to attract discerning consumers and retailers. This certification signals to the market that a company prioritizes safety and excellence, particularly through the use of cGMP supplements, resulting in higher sales and customer loyalty.
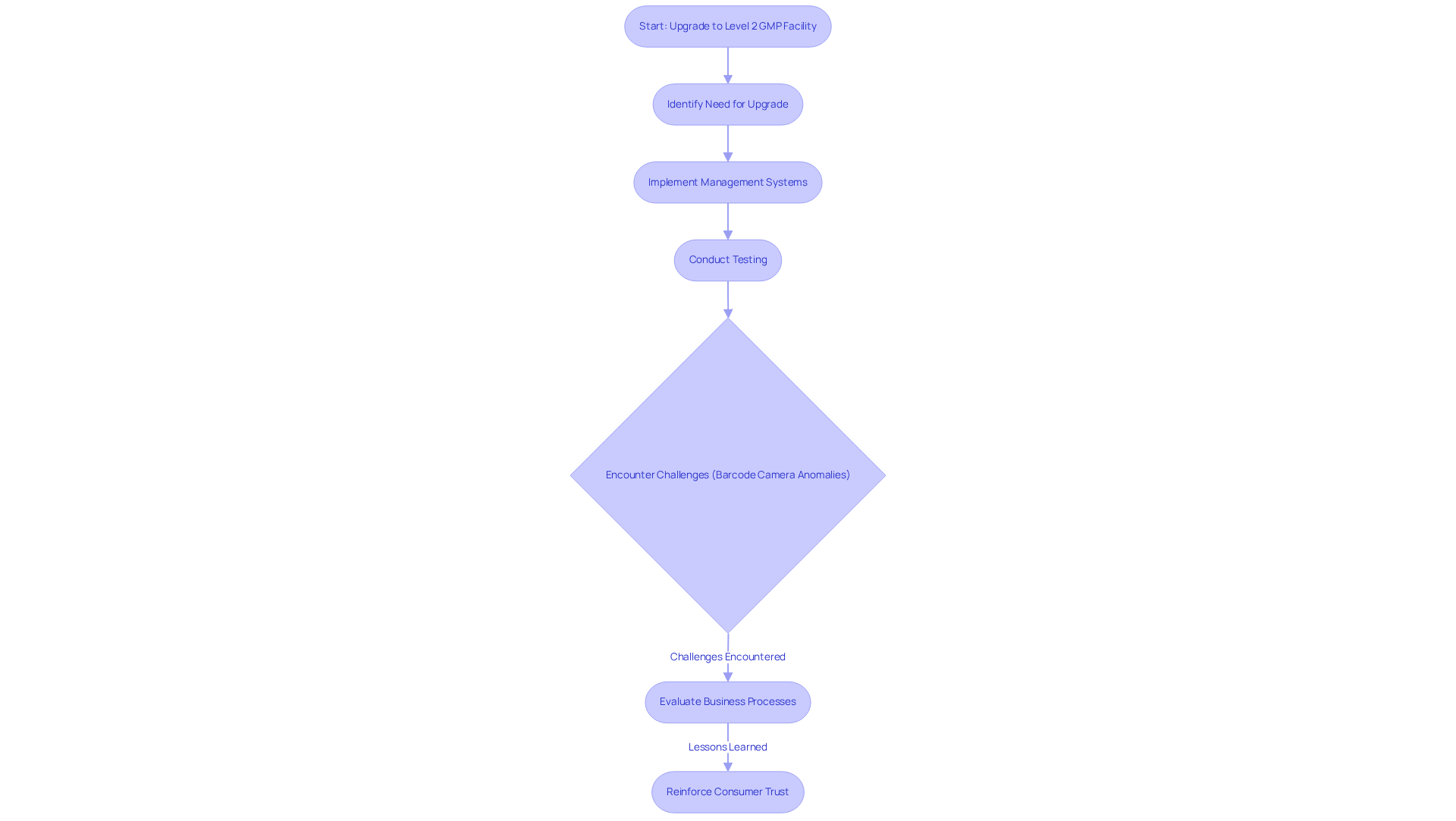
For example, AVS Life Sciences recently assisted a leading biotechnology company in upgrading its manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. This successful transition not only ensured compliance with stringent regulatory standards but also enhanced the client's capacity to produce targeted antibodies for cancer.
Throughout this process, AVS Life Sciences maintained a focus on , identifying challenges such as anomalies in test results caused by improperly installed barcode scanner cameras. The insights gained prompted the QC laboratory team to evaluate their business procedures, ultimately reinforcing the importance of cGMP supplements in enhancing a company's reputation and operational effectiveness within the competitive landscape of life sciences.
Improves Operational Efficiency in Manufacturing Processes
The use of cGMP supplements significantly enhances operational efficiency in manufacturing processes. By standardizing procedures and ensuring comprehensive training for all employees in best practices, companies can effectively reduce waste, minimize errors, and boost productivity with the use of cGMP supplements. A prime illustration of this is AVS Life Sciences' successful enhancement of a pharmaceutical manufacturer's facility from Biosafety Level 1 to Level 2 GMP standards for lentivirus production. This transformative project not only ensured adherence to stringent but also optimized the manufacturing process, demonstrating that such upgrades can lead to substantial reductions in operational costs and accelerate time-to-market for new products. By partnering with AVS Life Sciences, companies can leverage expert solutions in GMP regulations, validation, and engineering, ultimately enhancing their operational efficiency. This partnership is not merely an option; it is a strategic imperative for those committed to excellence in compliance and operational performance.
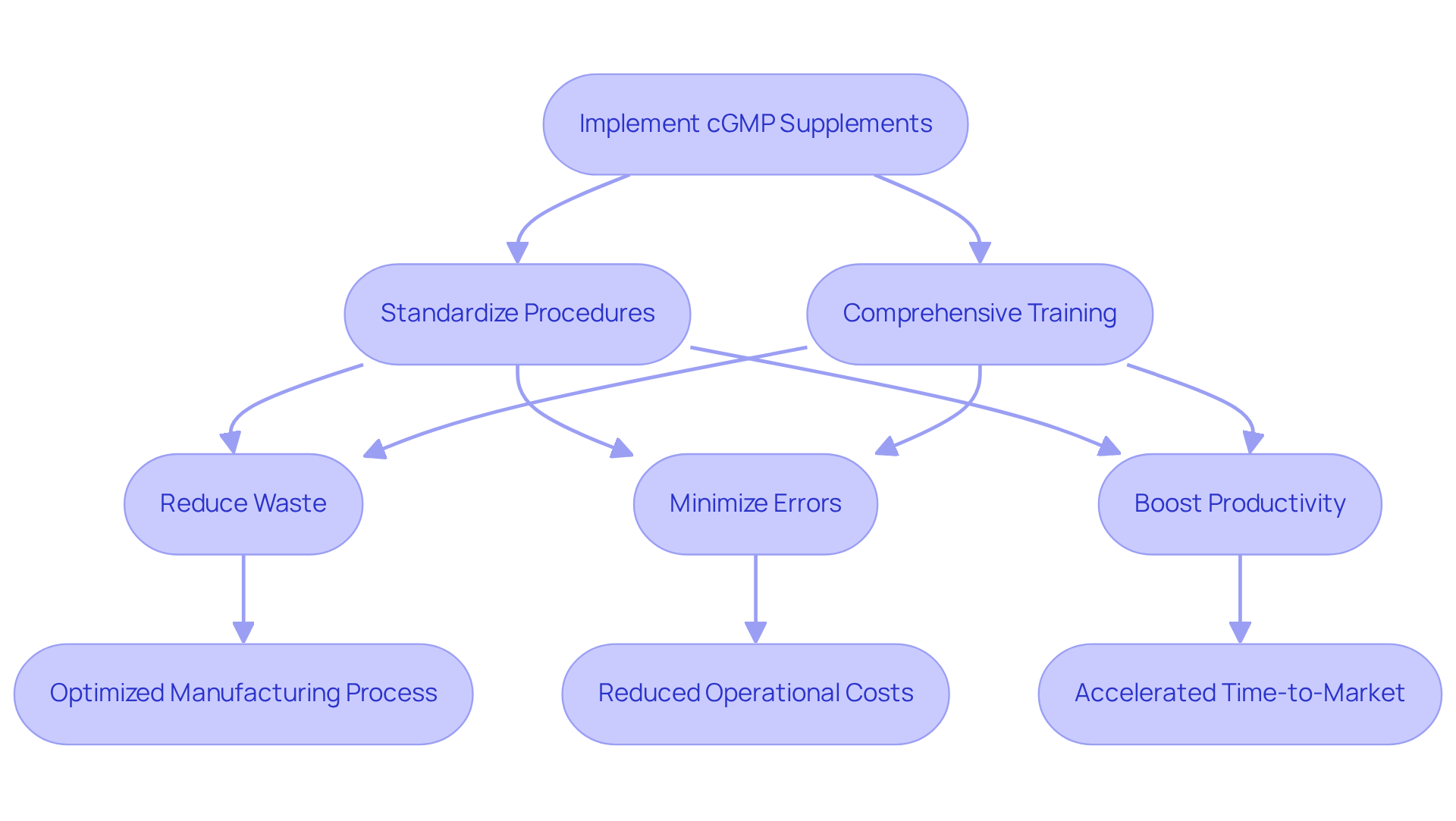
Enhances Brand Reputation and Consumer Trust
The use of cGMP supplements significantly enhances brand reputation and fosters consumer trust. When consumers recognize that cGMP supplements meet , they are more likely to choose that brand over its rivals. This trust arises from the confidence that the cGMP supplements are safe, effective, and of high standard, ultimately leading to increased repeat purchases and favorable word-of-mouth recommendations.
A transformative case study from AVS Life Sciences illustrates this point effectively. By aiding a prominent biotechnology firm in improving their production area from a Biosafety Level 1 GMP facility to a Level 2 GMP facility, AVS Life Sciences showcased how management systems and regulatory adherence solutions can greatly improve operational capabilities.
During this upgrade, challenges such as anomalies in test results were encountered, revealing that some barcode scanner cameras were installed upside down. This oversight was due to the scanner returning sets of values that could yield identical results, leading to unreliable test outcomes. The lessons learned from this experience prompted the QC laboratory team and Quality team to evaluate their business processes, ultimately reinforcing consumer trust in their products.
The successful conclusion of this project not only guaranteed adherence but also enabled the client to concentrate on creating medicines that enhance patient quality of life.
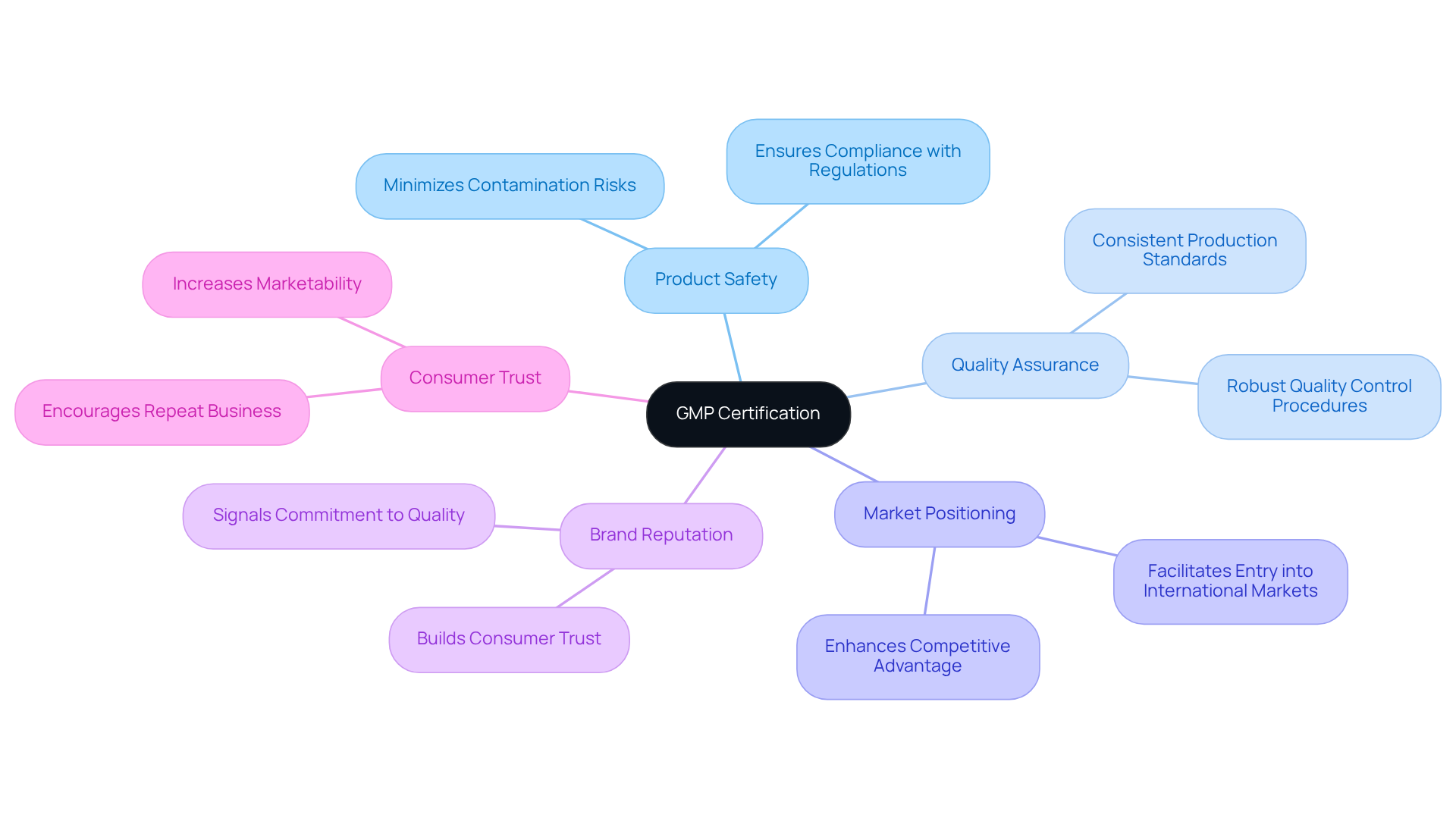
Unlocks Global Market Opportunities for Certified Products
Good Manufacturing Practice (GMP) certification serves as a vital gateway for dietary supplement producers aiming to penetrate global markets. Many nations require strict adherence to GMP standards for imported products, making this certification indispensable for businesses aspiring to achieve global growth.
By complying with these rigorous standards, manufacturers not only guarantee product safety and quality but also strategically position themselves in competitive markets. This compliance significantly enhances their marketability, as numerous retailers and consumers prefer or mandate that suppliers possess .
As a result, certified companies unlock lucrative opportunities, bolster their brand reputation, and cultivate consumer trust, ultimately propelling growth in the global marketplace.
AVS Life Sciences offers comprehensive consulting services designed to assist manufacturers in achieving current Good Manufacturing Practice compliance, ensuring they meet the essential standards necessary for success in the global market.
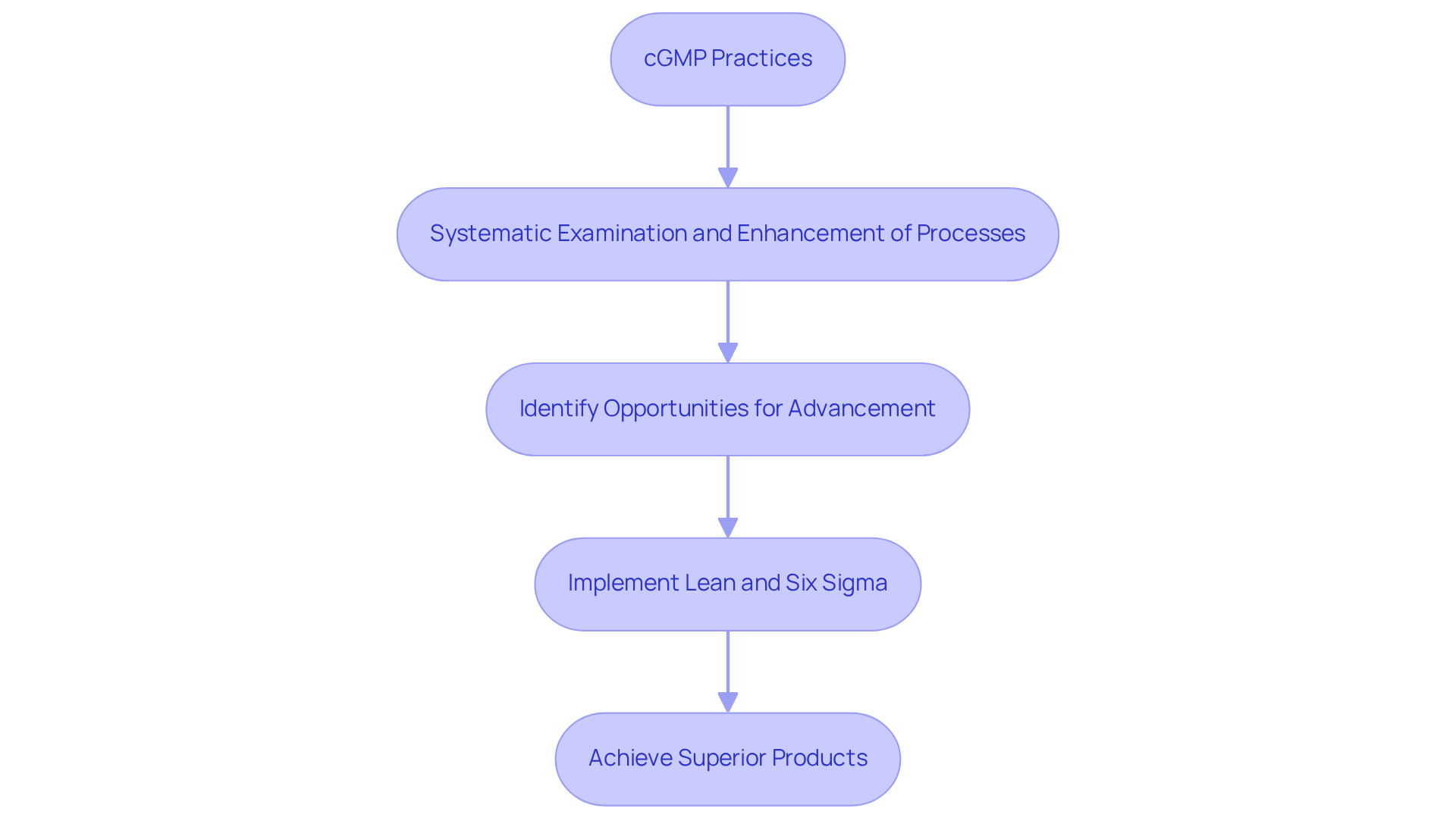
Encourages Continuous Improvement in Quality Practices
cGMP practices foster a robust culture of within organizations. By systematically examining and enhancing processes, companies can identify opportunities for advancement, leading to superior products. This proactive approach not only ensures compliance with regulatory standards but also empowers organizations to adapt to shifting consumer expectations and evolving regulations.
Implementing methodologies such as Lean and Six Sigma can significantly enhance operations, optimizing results and reinforcing a commitment to excellence. As W. Edwards Deming emphasized, knowing what to do is essential for achieving optimal outcomes; this principle is vital in cultivating a culture where excellence is a collective responsibility across all levels of the organization.
For further insights, consult the user manuals and FAQs provided by AVS Life Sciences, which offer practical guidance on effectively implementing these best practices.
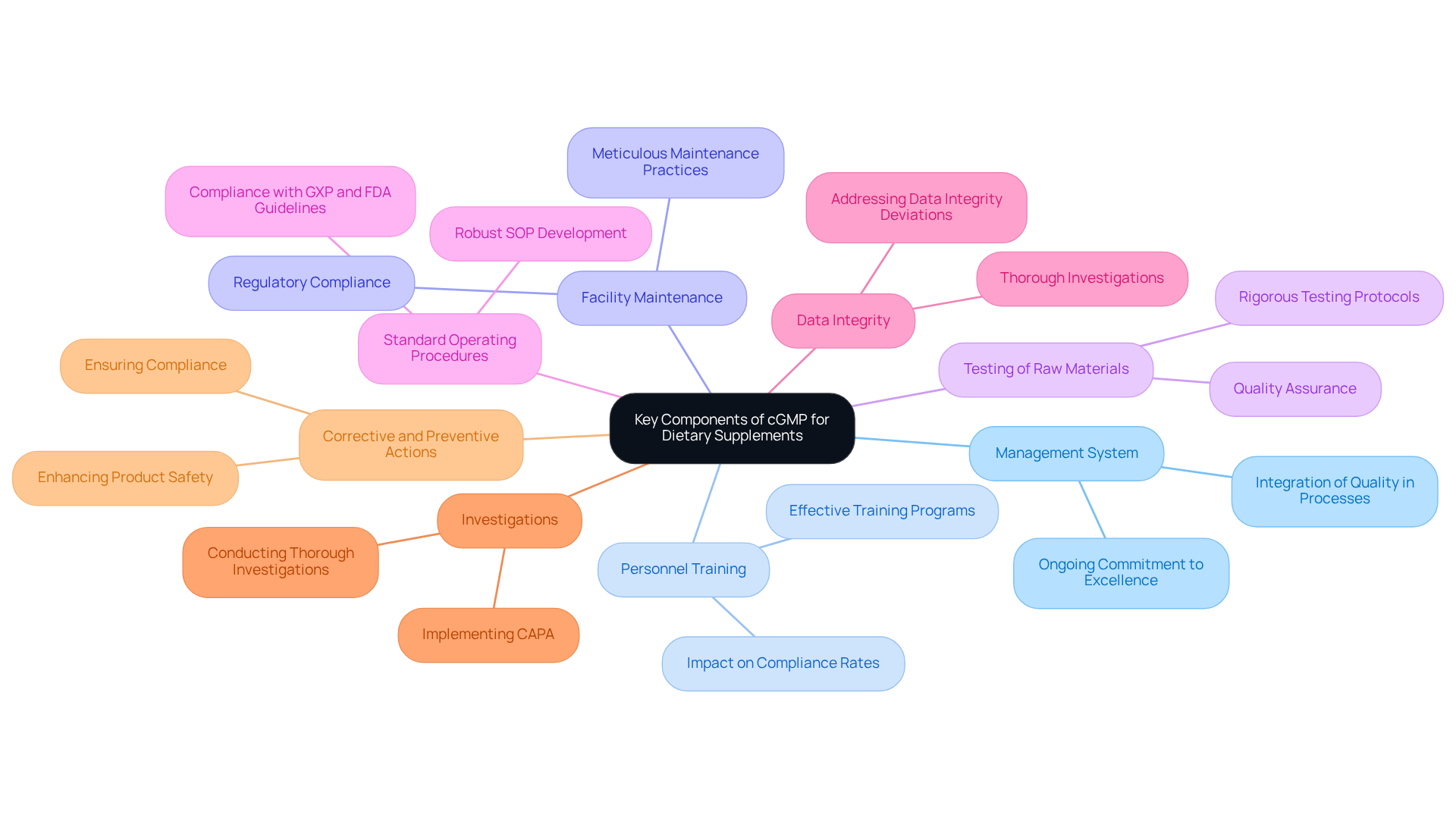
Key Components of cGMP for Dietary Supplements
The essential elements of cgmp supplements for dietary products encompass a , effective personnel training, meticulous facility and equipment maintenance, and rigorous testing of raw materials. Manufacturers must create robust standard operating procedures (SOPs) to uphold consistent standards and comply with regulatory requirements, including GXP and FDA guidelines.
Addressing Data Integrity Deviations, conducting thorough investigations, and implementing Corrective and Preventive Actions (CAPA) are critical for ensuring compliance and enhancing product safety. As industry leaders emphasize, excellence in management is not merely a checklist; it represents an ongoing commitment to superiority. Harold F. Dodge's assertion that excellence cannot be inspected into a product but must be integrated into every process underscores this point.
Furthermore, effective training programs are vital, with research indicating that well-trained staff can improve compliance rates by as much as 30%, significantly enhancing operational efficiency in regulated environments. By prioritizing these components and fostering a culture of shared responsibility for excellence throughout the organization, manufacturers can ensure that their products meet the highest standards of performance and safety, ultimately cultivating customer trust and satisfaction.
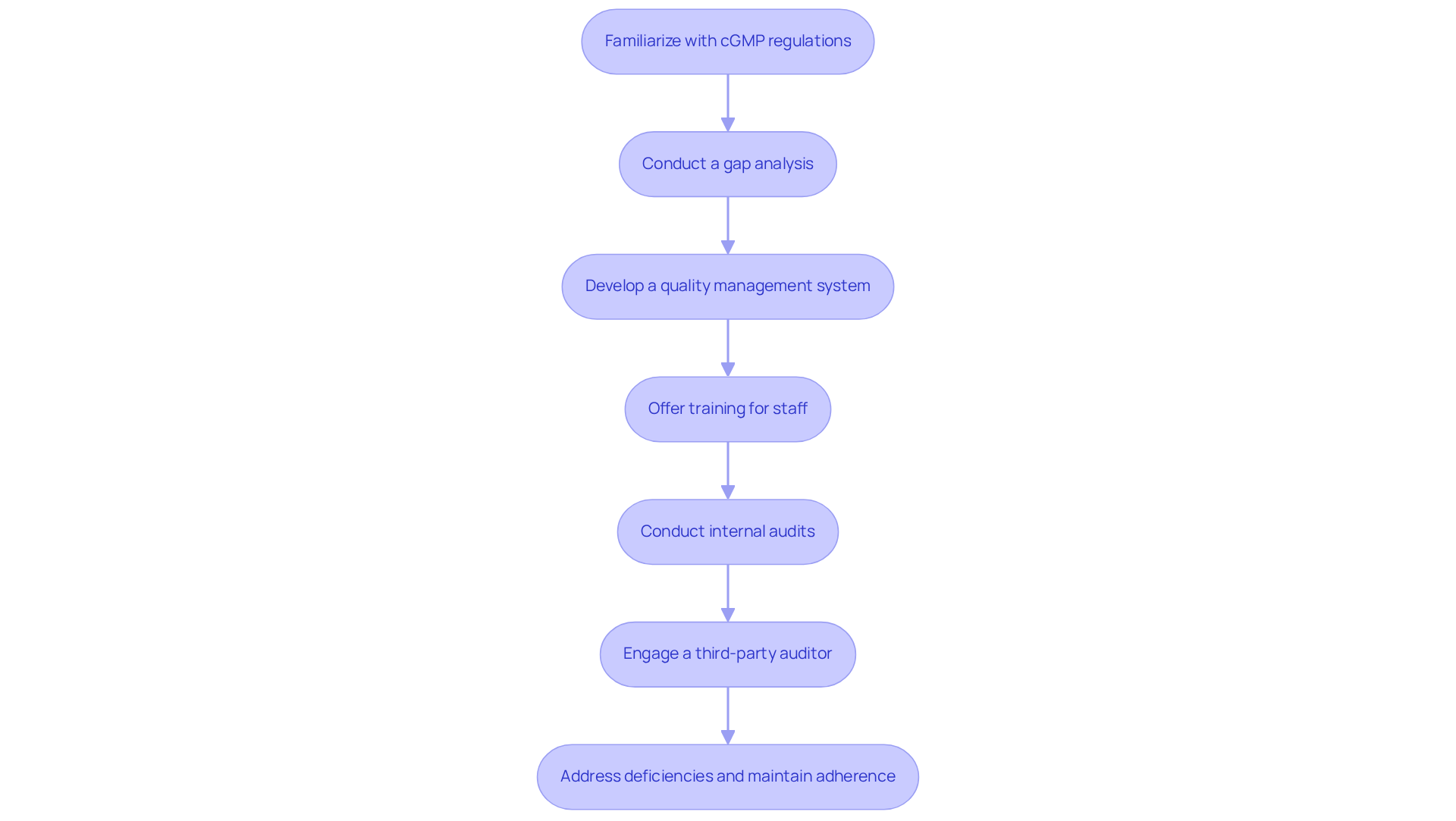
Steps to Obtain cGMP Certification for Dietary Supplements
To achieve , manufacturers must navigate a series of essential steps.
- First, they should familiarize themselves with cGMP supplements and the relevant regulations.
- Next, conducting a thorough gap analysis is crucial to identify areas that need improvement.
- Following this, developing and implementing a robust quality management system that aligns with regulatory standards is imperative.
- Additionally, offering comprehensive training for staff on good manufacturing practices related to cGMP supplements ensures understanding and adherence.
- Regular internal audits are necessary to verify compliance with established protocols.
- Engaging a qualified third-party auditor will assess readiness for certification.
- Any identified deficiencies must be promptly addressed, and ongoing adherence should be maintained through continuous reviews and updates.
For tailored consulting solutions related to cGMP compliance, consider reaching out to AVS Life Sciences. This structured approach not only facilitates successful certification but also fosters a culture of quality and accountability within the organization.
Conclusion
The significance of cGMP supplements in the dietary supplement industry cannot be overstated. By adhering to current Good Manufacturing Practices, companies not only ensure the safety and efficacy of their products but also build a foundation for trust and credibility among consumers. This commitment to quality management is essential for businesses looking to thrive in a competitive market, as it reflects a dedication to excellence and regulatory compliance.
Key benefits of cGMP supplements include:
- Enhanced product quality and safety
- Improved operational efficiency
- Increased consumer trust
Noteworthy examples, such as AVS Life Sciences' successful projects, illustrate how effective management systems can lead to significant operational improvements and compliance with rigorous regulatory standards. Furthermore, the potential for cost savings and competitive advantages through cGMP certification underscores the financial and reputational benefits that come with prioritizing quality practices.
In a landscape where consumer safety and regulatory compliance are paramount, the adoption of cGMP standards is not merely a recommendation but a necessity for dietary supplement manufacturers. By embracing these practices, companies can unlock global market opportunities, foster continuous improvement, and ultimately deliver superior products that enhance consumer health and well-being. The call to action is clear: prioritize cGMP compliance to not only meet regulatory requirements but to set a benchmark for quality and safety in the dietary supplement industry.
Frequently Asked Questions
What services does AVS Life Sciences provide for cGMP supplements?
AVS Life Sciences offers a comprehensive suite of services designed to ensure dietary supplements comply with current Good Manufacturing Practices (cGMP). Their expertise includes validation, engineering support, quality assurance consulting, and guidance on regulatory submissions.
Why is cGMP certification important for dietary supplement manufacturers?
cGMP certification is essential as it ensures products are manufactured in a controlled environment, reducing the risk of contamination and errors. It guarantees the identity, strength, and purity of supplements, enhancing the manufacturer's credibility and fostering consumer trust.
What recent trends are influencing the dietary supplement industry regarding cGMP?
There is a heightened awareness of the importance of cGMP supplements and certification, with all contracts signed requiring Quality Management System (QMS) certification. This trend emphasizes the need for stringent management practices to mitigate non-compliance risks.
How can AVS Life Sciences assist companies in enhancing their manufacturing practices?
AVS Life Sciences tailors solutions to assist clients in navigating regulatory complexities, providing personalized guidance throughout the compliance journey and helping organizations implement effective management systems.
What impact does cGMP compliance have on operational outcomes?
Effective implementation of cGMP practices can lead to significant improvements in operational outcomes, as demonstrated by AVS Life Sciences' successful project with a biotechnology company that upgraded its GMP facility on time and within budget.
What are the consequences of failing to adhere to cGMP standards?
Non-compliance with cGMP standards can lead to substantial fines, product recalls, and detrimental health consequences for consumers, highlighting the importance of maintaining rigorous quality management frameworks.
How does AVS Life Sciences support clients in maintaining regulatory compliance?
AVS Life Sciences provides customized consulting solutions to help clients navigate the complexities of regulatory requirements, ensuring adherence to FDA guidelines and minimizing the risk of regulatory breaches.
What financial benefits can companies achieve by prioritizing cGMP practices?
Companies that prioritize cGMP practices can achieve significant cost savings, with potential savings of an average of $1.25 million in compliance costs by appointing a dedicated compliance leader.
