10 Benefits of eCOA in Clinical Trials for Enhanced Compliance

Overview
The article underscores ten significant benefits of employing electronic Clinical Outcome Assessments (eCOA) in clinical trials. It emphasizes:
- Enhanced compliance through improved data accuracy
- Increased patient engagement
- Real-time monitoring
Evidence supports that eCOA systems effectively:
- Streamline data management
- Alleviate administrative burdens
- Promote patient adherence
Ultimately, these factors contribute to more efficient and reliable clinical study outcomes, reinforcing the value of integrating eCOA into clinical practices.
Introduction
The landscape of clinical trials is experiencing a significant transformation, propelled by the rising adoption of electronic Clinical Outcome Assessments (eCOA). This innovative approach not only tackles compliance challenges but also enhances data accuracy, fosters patient engagement, and boosts overall trial efficiency. As the industry shifts towards digital solutions, a pivotal question emerges: how can eCOA systems fundamentally reshape the conduct of clinical research, ensuring both regulatory adherence and enriched participant experiences? Delving into the myriad benefits of eCOA uncovers a pathway to more effective and reliable clinical trials, positioning it as an essential consideration for researchers and sponsors alike.
AVS Life Sciences: Enhanced Compliance and Quality Management through eCOA
addresses critical compliance challenges in research studies through the utilization of [electronic Clinical Outcome Evaluations](http://avslifesciences.com/blog-post/10-ctms-clinical-trial-benefits-for-compliance-officers), significantly enhancing adherence and quality oversight. By seamlessly integrating electronic Clinical Outcome Assessment into their operational framework, AVS ensures alignment with Good Manufacturing Practices (GMP) and essential regulatory standards, including GXP and FDA regulations. This strategic approach simplifies data management and underscores how AVS Life Sciences achieves high-quality standards through rigorous compliance with these regulations throughout the research lifecycle.
Industry specialists note that electronic clinical outcome assessments enable real-time monitoring and compliance tracking, vital for achieving favorable study outcomes. As the clinical trial landscape evolves, the adoption of electronic Clinical Outcome Assessments is becoming increasingly crucial. Trends indicate a shift towards more integrated and standardized solutions that prioritize regulatory compliance and enhance patient engagement.
Improved Data Accuracy: Ensuring Reliable Clinical Trial Outcomes with eCOA
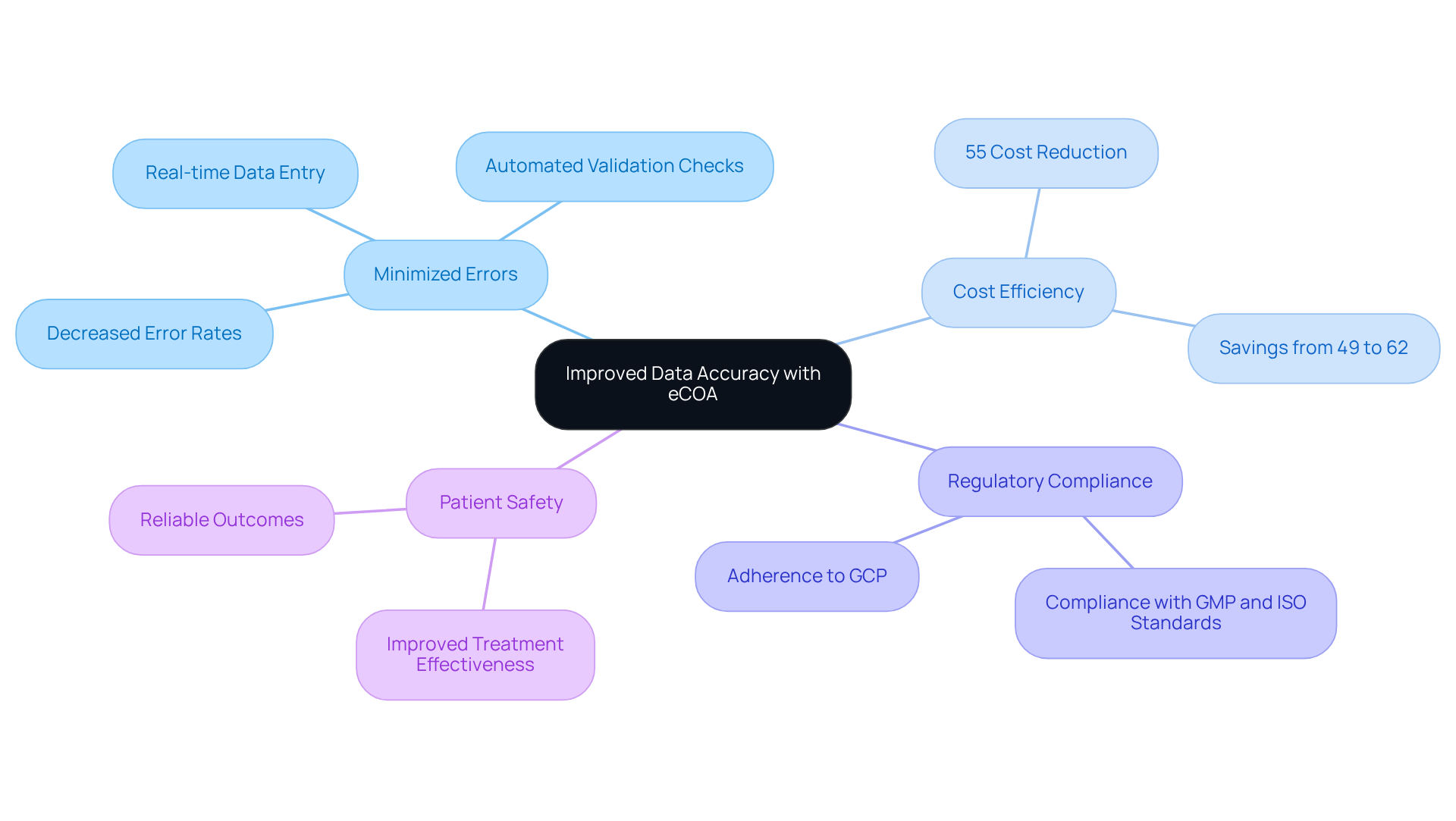
The electronic Clinical Outcome Assessment significantly enhances information precision by minimizing common errors associated with traditional paper-based collection methods. By leveraging features such as real-time data entry and automated validation checks, eCOA guarantees that the information captured is both complete and accurate. This level of precision is crucial for and for making informed decisions throughout the process.
Recent studies indicate that the electronic data collection (EDC) process can reduce collection costs by up to 55% compared to traditional paper-based methods, underscoring its efficiency. Experts in research emphasize that enhanced data accuracy not only yields more reliable outcomes but also improves patient safety and treatment effectiveness.
Furthermore, the transition from paper-based case report forms (CRF) to electronic CRF has shown a notable decrease in error rates, bolstering the reliability of electronic assessments in clinical settings. A case study by AVS Life Sciences revealed that during their upgrade of a biotechnology GMP facility, the implementation of electronic Clinical Outcome Assessments in ecoa clinical trials not only improved adherence to Good Clinical Practice (GCP) but also uncovered critical insights regarding quality assurance procedures.
This experience underscored the necessity of addressing gaps in testing protocols and promoting open communication within teams. As the industry continues to evolve, the adoption of ecoa clinical trials is vital for ensuring compliance and achieving favorable study results.
Increased Patient Engagement: Boosting Participation and Data Quality with eCOA
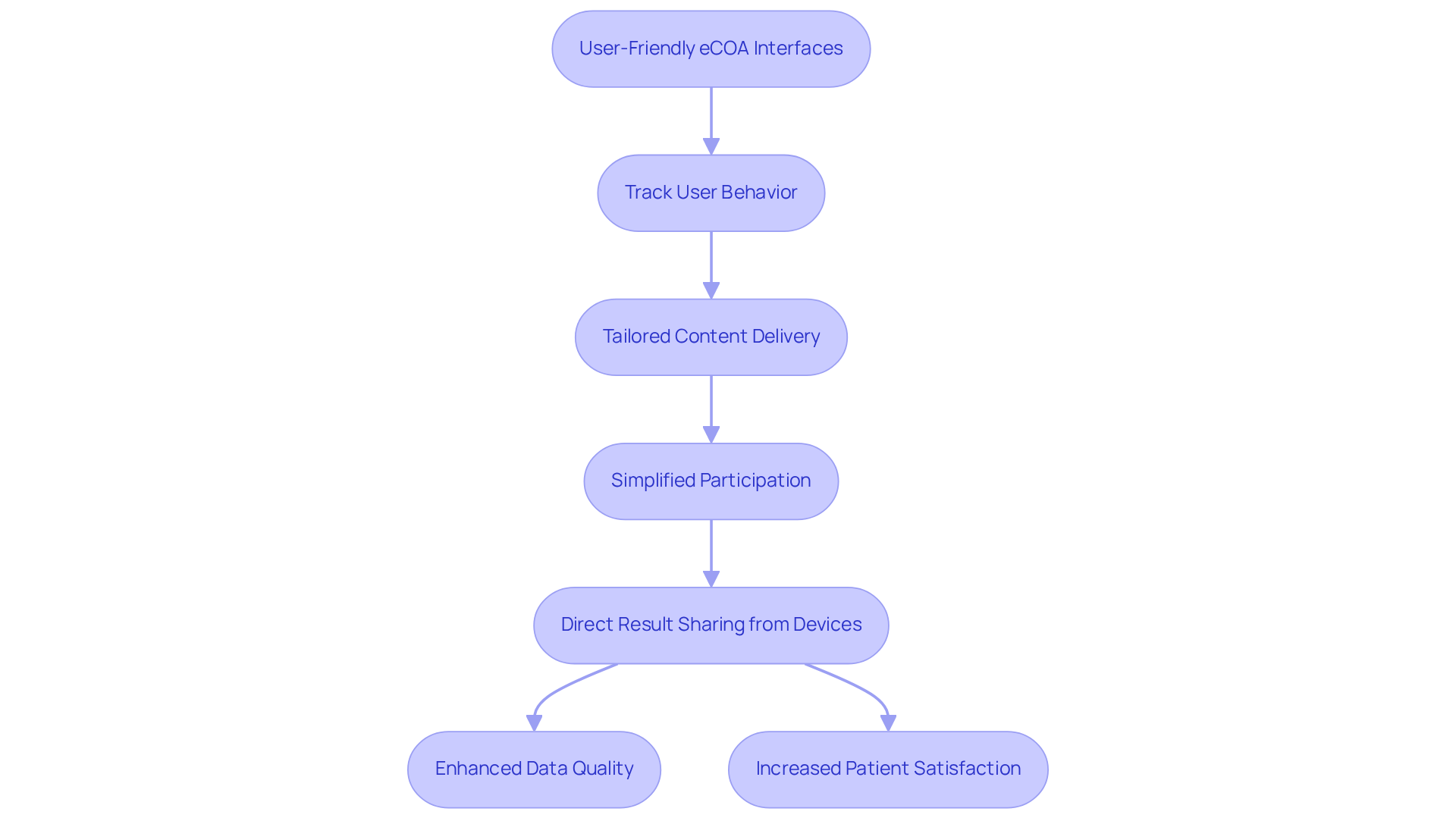
eCOA platforms are designed to enhance patient involvement through user-friendly interfaces and convenient information entry options. AVS Life Sciences meticulously tracks user behavior on its platforms to discern which services resonate most with patients. This insight allows for the delivery of tailored content and advertising, ensuring that patients receive information pertinent to their interests. Such a data-driven approach simplifies participation, making it more accessible.
As a result, patients can share results directly from their devices, which not only elevates the quality of information but also boosts patient satisfaction and retention rates in ecoa clinical trials. When patients feel more , they are significantly more likely to comply with study protocols and provide accurate information, thereby enhancing the overall integrity of the research.
Real-Time Data Collection: Streamlining Clinical Trials with eCOA
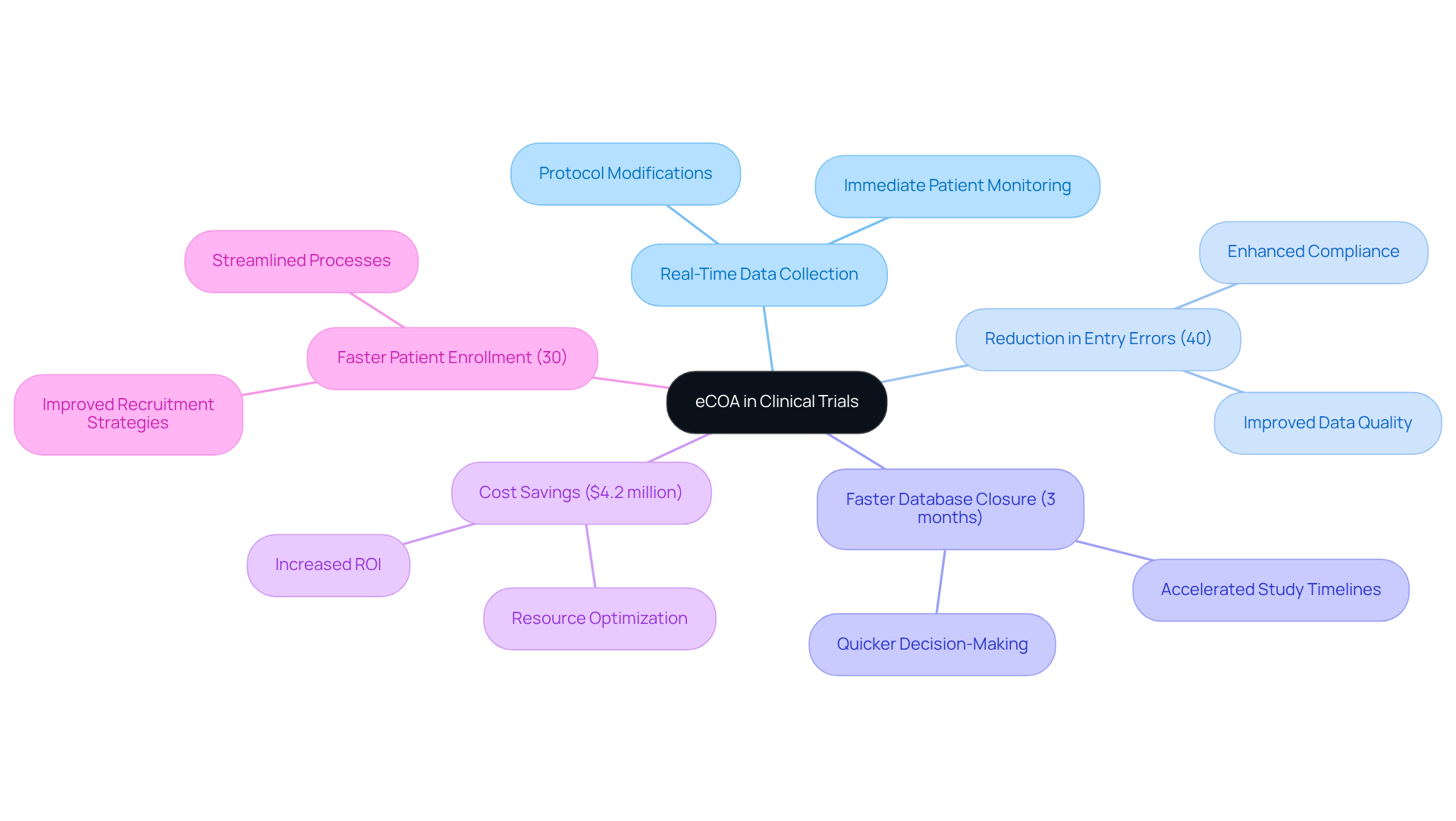
eCOA's remarkable characteristic lies in its capacity for , empowering clinical research teams to observe patient reactions and maintain information integrity as events unfold. This immediacy allows for swift modifications to research protocols, significantly reducing the delays associated with traditional information gathering techniques. As a result, decision-making is expedited, leading to enhanced efficiency and compliance with GXP standards.
For instance, a leading pharmaceutical firm that optimized a Phase III oncology study through a unified information management system achieved:
- A 40% reduction in entry errors
- A three-month acceleration in time to database closure
- Realized savings of $4.2 million
These advancements underscore the critical role of real-time information monitoring in sustaining the momentum of research studies and ensuring that information remains relevant and actionable.
Furthermore, research from the Clinical Trials Efficiency Report indicates that studies utilizing integrated, automated data management workflows complete patient enrollment 30% faster and attain database lock in 45% less time compared to those relying on fragmented systems. This highlights the transformative impact of electronic outcome assessments on study results within eCOA clinical trials, positioning them as essential tools for enhancing compliance and effectiveness.
To leverage these benefits and fulfill GXP sponsor obligations, pharmaceutical compliance officers should consider the integration of eCOA clinical trials in their research studies, thereby ensuring effective oversight of service providers.
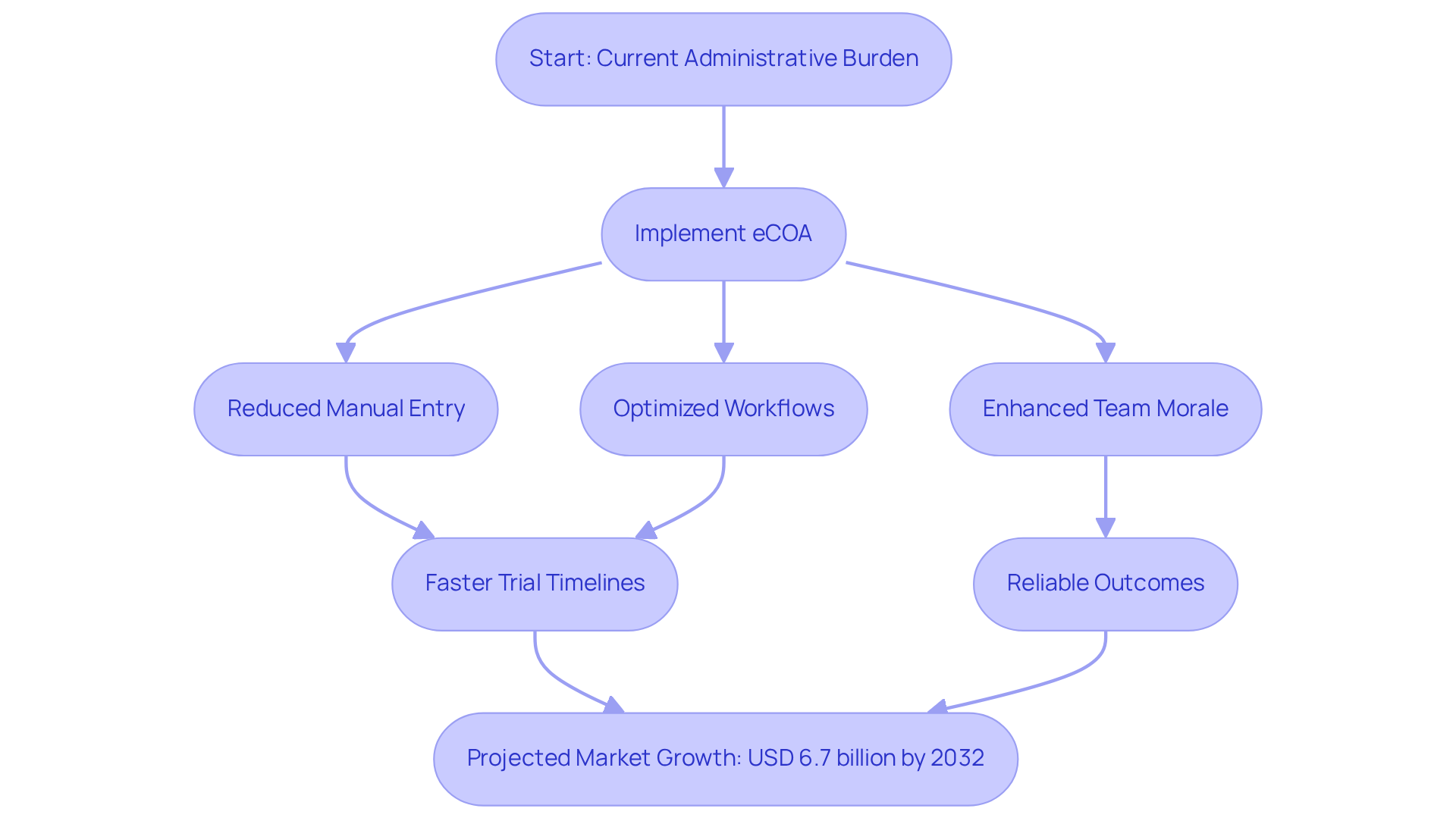
Reduced Administrative Burden: Simplifying Clinical Trial Management with eCOA
The electronic outcome assessment significantly reduces the administrative load on research teams by automating information gathering and management procedures. This automation greatly decreases the need for manual information entry, thereby lowering the chance of mistakes and enabling staff to concentrate their time and resources on more essential tasks. By optimizing workflows, the electronic Clinical Outcome Assessment empowers clinical teams to prioritize patient care and information analysis over administrative duties.
Industry experts assert that this enhanced efficiency not only boosts team morale but also accelerates the overall trial timeline, leading to quicker and more reliable outcomes. Furthermore, the integration of electronic Clinical Outcome Assessments with other eClinical technologies facilitates seamless data exchange and real-time oversight, essential for ensuring compliance and enhancing study implementation.
As a leader in quality management and regulatory compliance, AVS Life Sciences plays a pivotal role in ensuring that electronic Clinical Outcomes Assessments align with industry standards and regulatory requirements. With the electronic Clinical Outcome Assessment market projected to reach USD 6.7 billion by 2032, its is unmistakable.
Enhanced Patient Compliance: Facilitating Adherence through eCOA
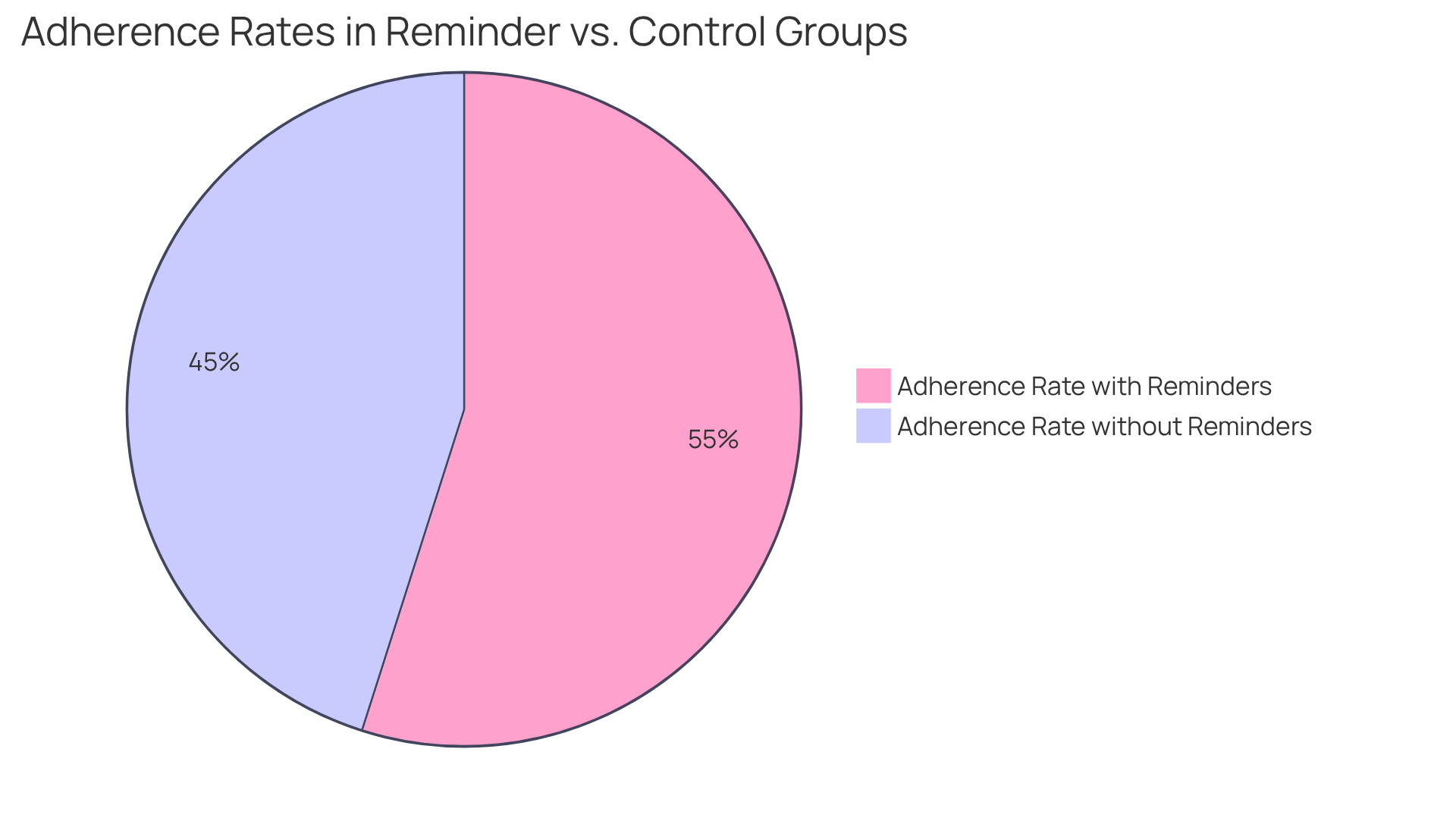
The electronic Clinical Outcome Assessment significantly enhances patient adherence by delivering timely notifications and facilitating straightforward information entry. Patients receive alerts on their devices, encouraging them to complete assessments on time. This proactive approach guarantees consistent and accurate .
By optimizing the reporting process, the system fosters a sense of accountability among participants, which is crucial for achieving higher compliance rates. Research demonstrates that reminder systems can yield adherence rates of 66.61% in groups receiving reminders, compared to 54.71% in control groups. The average adherence rate for reminder groups stands at 66.61%, with a standard deviation of 15.93%, indicating variability in outcomes.
Furthermore, the integration of electronic clinical outcome assessments has been shown to significantly improve adherence, with data revealing that when reminders are employed, 15.6% fewer individuals neglect their medication. This approach not only enhances the reliability of study data but also promotes a more engaged participant experience in ecoa clinical trials, ultimately contributing to the success of medical studies.
According to the World Health Organization, only 50% of patients adhere to chronic therapy, underscoring the importance of electronic Clinical Outcome Assessments in addressing these challenges. The authors noted that electronic reminders are straightforward to implement and should be integrated into standard care for patients on long-term medication.
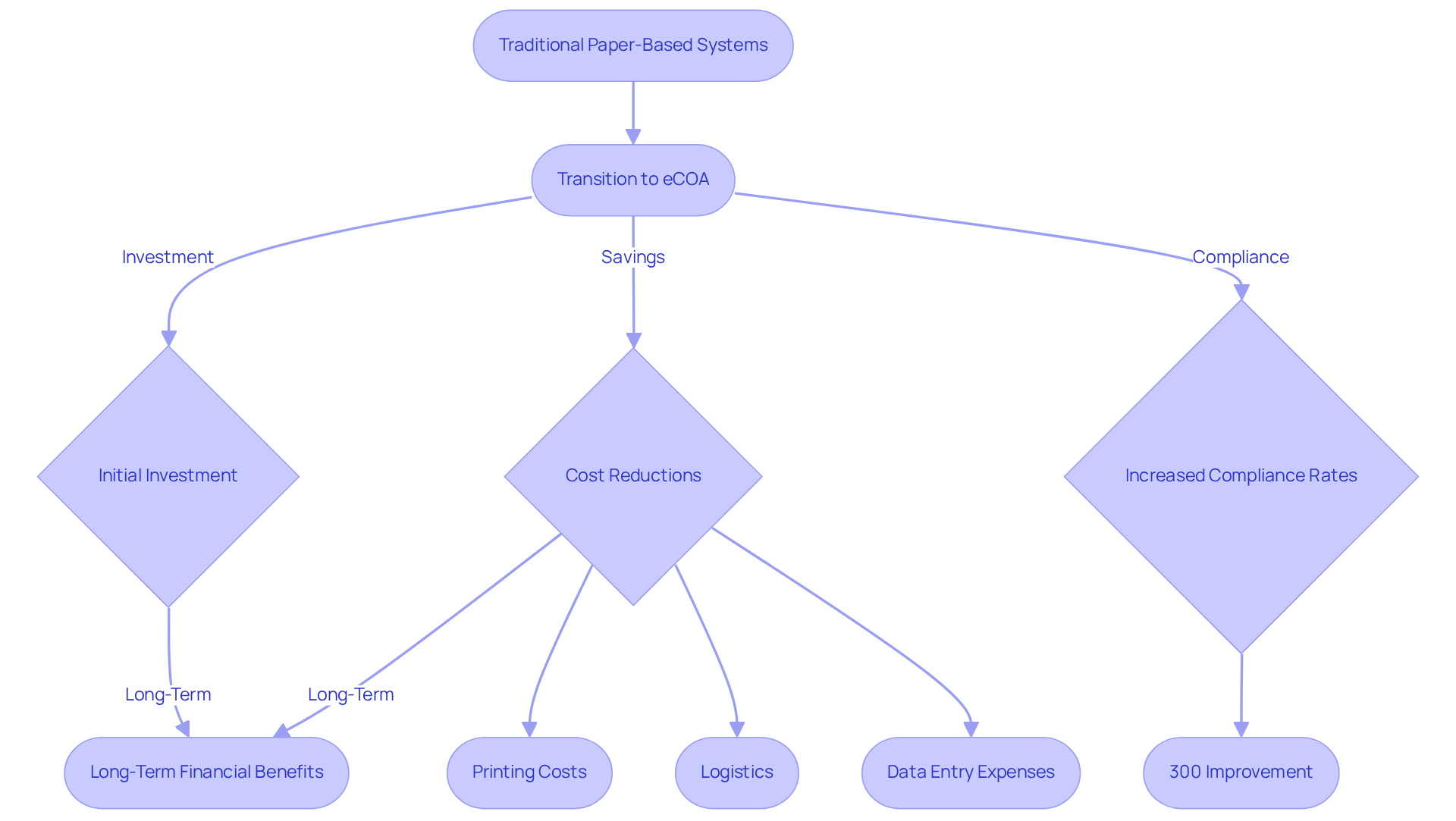
Cost-Effectiveness: Maximizing Resources with eCOA in Clinical Trials
Utilizing electronic Clinical Outcome Evaluations can lead to substantial cost reductions in clinical studies. Transitioning from traditional paper-based systems to electronic clinical outcome assessments allows organizations to significantly lower operational expenses related to printing, logistics, and manual information entry. For instance, a mid-sized study involving 1,000 patients across 50 locations can save hundreds of thousands of dollars by implementing electronic clinical outcome assessments, effectively eliminating hidden costs associated with paper management, including printing and data entry expenses.
The efficiency gained through real-time information gathering and optimized procedures not only reduces costs but also accelerates testing schedules. Clinical trials employing electronic Clinical Outcome Assessments have demonstrated compliance rates that are 300% higher than those relying on paper methods, underscoring the effectiveness of electronic assessments in enhancing data quality and operational efficiency (source: external data).
Experts in the field assert that while the initial investment in electronic Clinical Outcome Assessments may seem higher, the long-term financial benefits far outweigh these costs. Branden Kusanto notes, "While the initial expense of adopting electronic Clinical Outcome Assessments may appear superficially greater than paper, the long-term advantages significantly surpass the early expenditure." By reducing the need for extensive data cleaning and management associated with paper systems, this solution empowers sponsors to allocate resources more effectively, ultimately yielding faster and more reliable outcomes. This financial impact positions eClinical Outcomes Assessment as a strategic choice for sponsors aiming to while upholding rigorous quality standards. Furthermore, electronic clinical outcome assessments align with the shift towards decentralized studies, enhancing accessibility and convenience for participants.
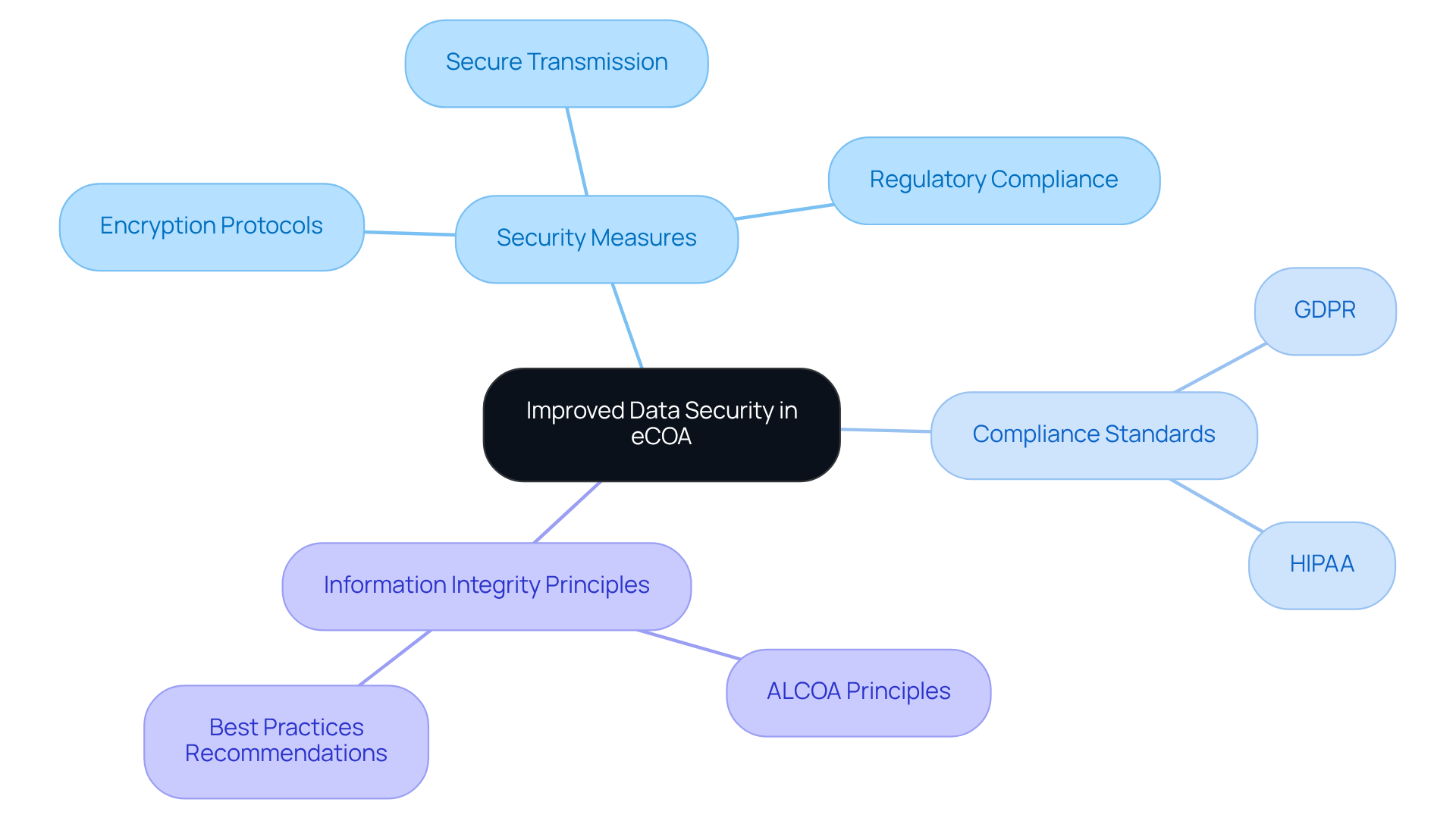
Improved Data Security: Safeguarding Patient Information with eCOA
eCOA systems integrate advanced security measures to safeguard patient information. These include:
- Encryption protocols
- Secure information transmission
- Compliance with stringent regulations such as GDPR and HIPAA
Such features not only ensure adherence to legal standards but also foster trust among participants, which is crucial for the integrity of eCOA clinical trials. AVS Life Sciences is dedicated to upholding privacy practices, ensuring that personal information is shared only with reliable partners who maintain confidentiality.
Experts emphasize that preserving information security is vital for upholding the credibility of research, especially in eCOA clinical trials, ensuring that patient details remain confidential and safeguarded throughout the study lifecycle. Moreover, the ALCOA principles—Attributable, Legible, Contemporaneous, Original, and Accurate—serve as a fundamental framework for ensuring information integrity in research studies. Recent updates from the ISPOR Task Force further highlight the importance of adhering to good practice recommendations for electronic clinical outcome assessment modifications. This reinforces the necessity for thorough training for site personnel on collection, aimed at reducing errors and improving compliance.
Scalability: Adapting eCOA Solutions for Diverse Clinical Trial Needs
Electronic clinical outcome assessment solutions exhibit remarkable adaptability, allowing for customization that meets the unique requirements of various clinical studies. Whether managing a small pilot study or a large multi-site trial, these can be tailored to accommodate diverse needs, including different data collection techniques and participant involvement strategies. This scalability ensures that sponsors can effectively implement electronic Clinical Outcome Assessments across a wide range of therapeutic areas and study designs. Notably, the electronic Clinical Outcome Assessment and electronic Source market is projected to reach USD 6.7 billion by 2032, underscoring the growing importance of these solutions in medical research.
The ability to develop standardized evaluation templates and workflows not only streamlines processes but also enhances data comparability and reliability across studies. A case study titled 'Standardization and Data Consistency Across Studies' illustrates how electronic Clinical Outcome Assessment software empowers sponsors to create reusable templates, meeting the critical requirement for consistency in clinical trials. Additionally, the findings from the case study 'Accelerating Electronic Clinical Outcome Assessments Trials for a Top-3 CRO and Top-10 Pharmaceutical Company' highlight how these assessments can mitigate challenges related to extended configuration timelines and variable study designs, facilitating more efficient study initiation.
Moreover, key features of electronic clinical outcome assessment software, such as real-time information capture and adherence monitoring, are essential for enhancing participant engagement and ensuring data accuracy. As the industry shifts towards digital solutions, the flexibility of electronic Clinical Outcome Assessment systems stands out as a significant advantage, ensuring that medical research can adapt to evolving demands and regulatory landscapes.
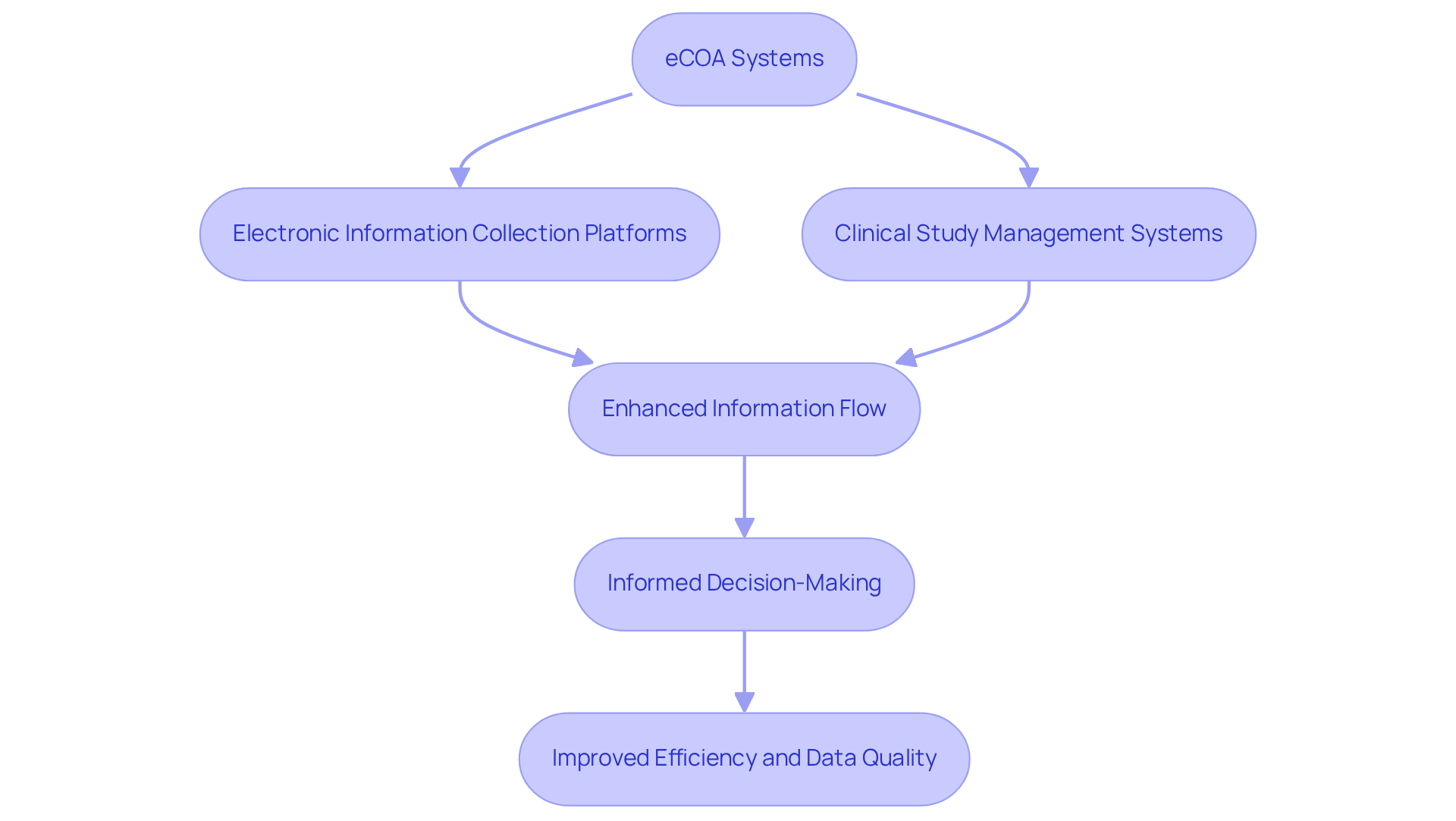
Integration Capabilities: Enhancing Clinical Trial Efficiency with eCOA
Electronic Clinical Outcome Assessment (eCOA) systems are designed to seamlessly integrate with various clinical research technologies, including electronic information collection platforms and clinical study management systems. This integration significantly enhances information flow, facilitating thorough analysis that ultimately in experiments.
By connecting diverse systems, the platform fosters a unified strategy for information management, enabling sponsors to leverage real-time insights for informed decision-making. Experts assert that such integration is crucial for optimizing operational processes and maintaining information integrity, an aspect that is increasingly vital as the sector shifts towards more effective and patient-centric approaches.
As Mike Hughes, Chief Product Officer, articulates, "Our revamped Automated Data Change Form as part of our 7.x electronic Clinical Outcome Assessment platform signifies a substantial improvement in managing information for research studies."
Furthermore, the adoption of connected devices in research studies has reached 36%, with current usage ranging from 28% to 46% across therapeutic fields, highlighting a growing trend towards incorporating innovative technologies that enhance patient engagement and streamline information gathering processes.
The FDA's recommendations on decentralized studies further bolster the integration of electronic data collection systems, underscoring the role of digital health technologies in improving study efficiency.
A case study on the advantages of connected devices illustrates how these technologies lead to enhanced data quality and increased patient involvement, demonstrating the tangible benefits of eCOA integration in eCOA clinical trials.
Conclusion
The implementation of electronic Clinical Outcome Assessments (eCOA) in clinical trials signifies a transformative shift towards enhanced compliance, improved data accuracy, and increased patient engagement. By leveraging advanced technologies, eCOA streamlines data collection and ensures adherence to regulatory standards, significantly bolstering the overall quality of clinical research.
Throughout the article, the numerous advantages of eCOA have been highlighted, including:
- Real-time data collection
- Reduced administrative burdens
- Enhanced patient compliance
- Cost-effectiveness
These factors collectively contribute to more reliable outcomes, as evidenced by case studies from AVS Life Sciences showcasing the successful integration of eCOA in various clinical settings. The adaptability of eCOA solutions for diverse clinical trial needs further underscores its importance in modern research, ensuring that studies can be tailored to meet specific requirements while maintaining high standards of data integrity and security.
As the clinical trial landscape continues to evolve, embracing eCOA is not merely an option but a necessity for organizations aiming to optimize their research processes. By prioritizing the integration of electronic assessments, sponsors can enhance patient engagement, streamline operations, and achieve more accurate results. The call to action is clear: adopting eCOA solutions is essential for any clinical trial striving to thrive in an increasingly competitive and regulated environment.
Frequently Asked Questions
What is the role of AVS Life Sciences in compliance and quality management?
AVS Life Sciences addresses compliance challenges in research studies by utilizing electronic Clinical Outcome Assessments (eCOA), which enhance adherence and quality oversight while ensuring alignment with Good Manufacturing Practices (GMP) and regulatory standards like GXP and FDA regulations.
How does eCOA improve data accuracy in clinical trials?
eCOA enhances data accuracy by minimizing errors associated with traditional paper-based collection methods through real-time data entry and automated validation checks, ensuring the information captured is complete and accurate.
What are the cost benefits of using electronic data collection (EDC) compared to traditional methods?
The electronic data collection process can reduce collection costs by up to 55% compared to traditional paper-based methods, highlighting its efficiency.
How does AVS Life Sciences enhance patient engagement through eCOA?
AVS Life Sciences enhances patient engagement by providing user-friendly eCOA platforms that allow convenient information entry and by tracking user behavior to deliver tailored content, making participation more accessible and relevant to patients.
What impact does increased patient engagement have on clinical trials?
Increased patient engagement leads to higher patient satisfaction and retention rates, as engaged patients are more likely to comply with study protocols and provide accurate information, thus enhancing the integrity of the research.
What insights did AVS Life Sciences gain from implementing eCOA in their clinical trials?
During an upgrade of a biotechnology GMP facility, AVS Life Sciences found that implementing eCOA improved adherence to Good Clinical Practice (GCP) and uncovered critical insights regarding quality assurance procedures, emphasizing the need for addressing gaps in testing protocols and promoting open communication within teams.
