10 Benefits of Continuous Emissions Monitoring for Pharma Compliance

Overview
The article emphasizes the critical role of continuous emissions monitoring (CEMS) in addressing compliance challenges within the pharmaceutical industry. By ensuring enhanced regulatory adherence, operational efficiency, and substantial cost savings, CEMS stands as a pivotal solution. Evidence indicates that CEMS not only facilitates real-time data collection but also significantly reduces the risk of non-compliance fines. Furthermore, it empowers companies to swiftly tackle environmental impacts, thereby promoting sustainability and ensuring workplace safety. Engaging with CEMS is not merely a regulatory obligation; it is a strategic move towards fostering a compliant and responsible business environment.
Introduction
The pharmaceutical industry faces mounting pressure to comply with stringent environmental regulations, making continuous emissions monitoring an essential tool for success. By implementing these systems, organizations not only ensure adherence to legal standards but also unlock a multitude of benefits, ranging from enhanced operational efficiency to significant cost savings. However, as the landscape of regulatory compliance evolves, pharmaceutical companies must navigate these complexities while maintaining a commitment to sustainability and safety. This article explores ten compelling advantages of continuous emissions monitoring, revealing how it can transform compliance efforts into a strategic advantage.
AVS Life Sciences: Comprehensive Regulatory Compliance Solutions for Continuous Emissions Monitoring
AVS Life Sciences provides comprehensive services tailored to ensure adherence to environmental regulations within the pharmaceutical sector. Their customized approach encompasses validation, quality assurance consulting, and engineering support, specifically designed to address the unique challenges faced by clients in . By leveraging their extensive expertise, AVS facilitates the implementation of effective continuous emissions monitoring systems that comply with stringent regulatory requirements. This guarantees accurate and efficient monitoring of discharges, which is essential for maintaining compliance and mitigating environmental impact.
Recent regulations have heightened the emphasis on emissions monitoring, with the U.S. EPA broadening reporting requirements to encompass small and mid-sized manufacturing facilities. In response, AVS Life Sciences has successfully aided numerous pharmaceutical firms in deploying continuous emissions monitoring systems, enabling them to meet these evolving standards. The integration of CEMS not only supports regulatory compliance but also enhances operational efficiency, as evidenced by the growing market for these systems, projected to reach USD 8.5 billion by 2033.
Industry leaders underscore the importance of adhering to environmental regulations, recognizing that effective monitoring of pollutants is vital for safeguarding public health and fostering sustainable practices. AVS Life Sciences sets itself apart as a dependable partner in this endeavor, assisting organizations in navigating the complexities of regulations while fostering a commitment to environmental stewardship. Client testimonials highlight AVS's thoroughness and professionalism, reinforcing their reputation as a premier provider of consulting and validation services in .
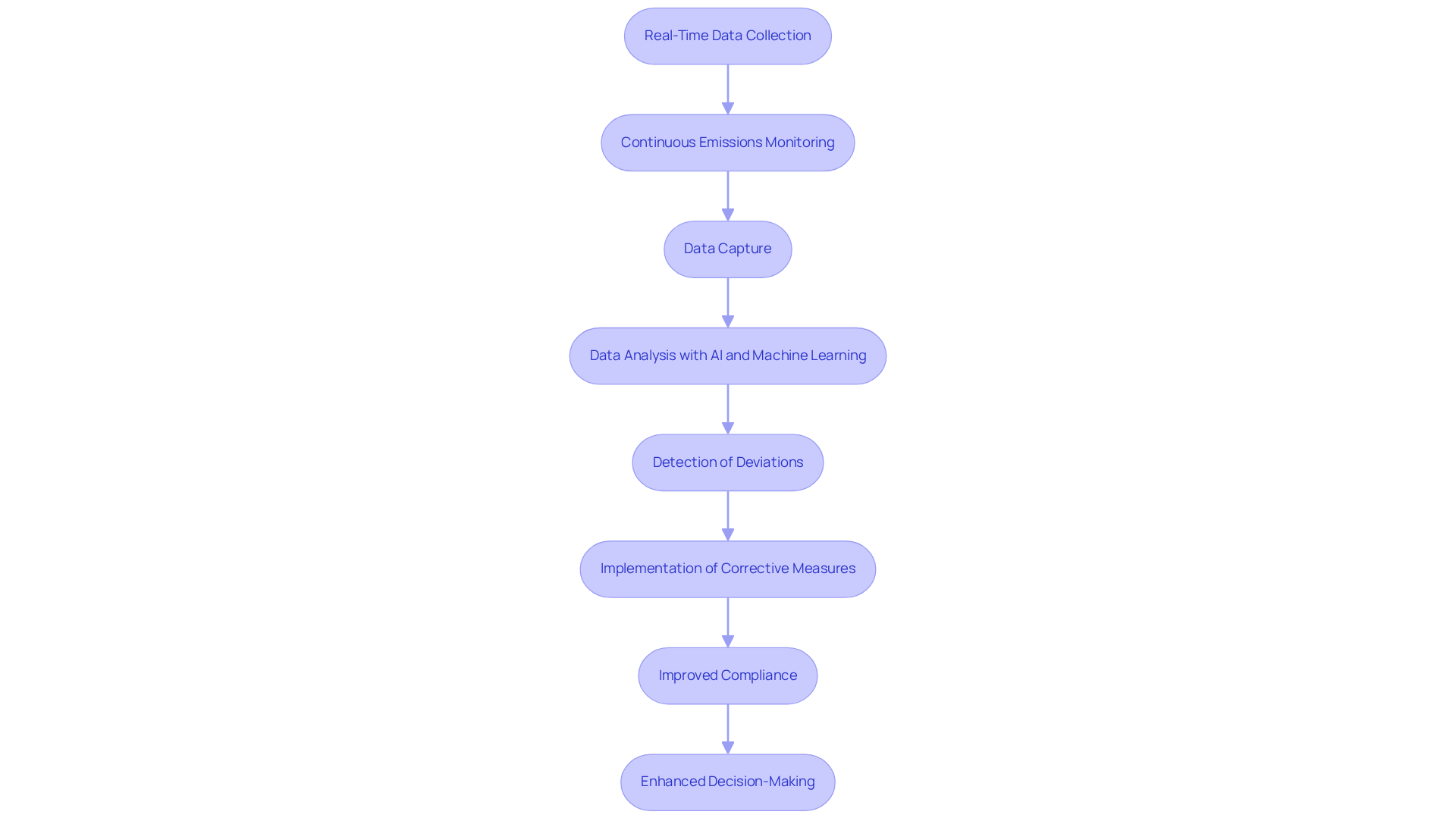
Real-Time Data Collection: Enhancing Decision-Making in Pharmaceutical Compliance
Ongoing monitoring systems, including continuous emissions monitoring, deliver immediate data that is essential for informed decision-making in pharmaceutical regulations. By utilizing continuous emissions monitoring to capture emissions data instantaneously, organizations can swiftly detect deviations from regulatory standards and implement corrective measures. This proactive strategy not only ensures adherence but also significantly enhances , enabling companies to address potential issues promptly.
Statistics indicate that companies utilizing fully integrated data frameworks experience decision-making processes that are 29% quicker, underscoring the critical role of real-time data in improving regulatory operations. Moreover, continuous emissions monitoring systems utilize AI and machine learning to analyze real-time data, facilitating the early identification of safety concerns and optimizing regulatory reporting.
Oversight officials have noted that access to real-time pollution data bolsters their ability to manage regulations effectively, leading to improved patient safety and reduced operational risks. Overall, the integration of real-time pollution data into decision-making processes is vital for maintaining high standards of compliance and operational excellence within the pharmaceutical sector.
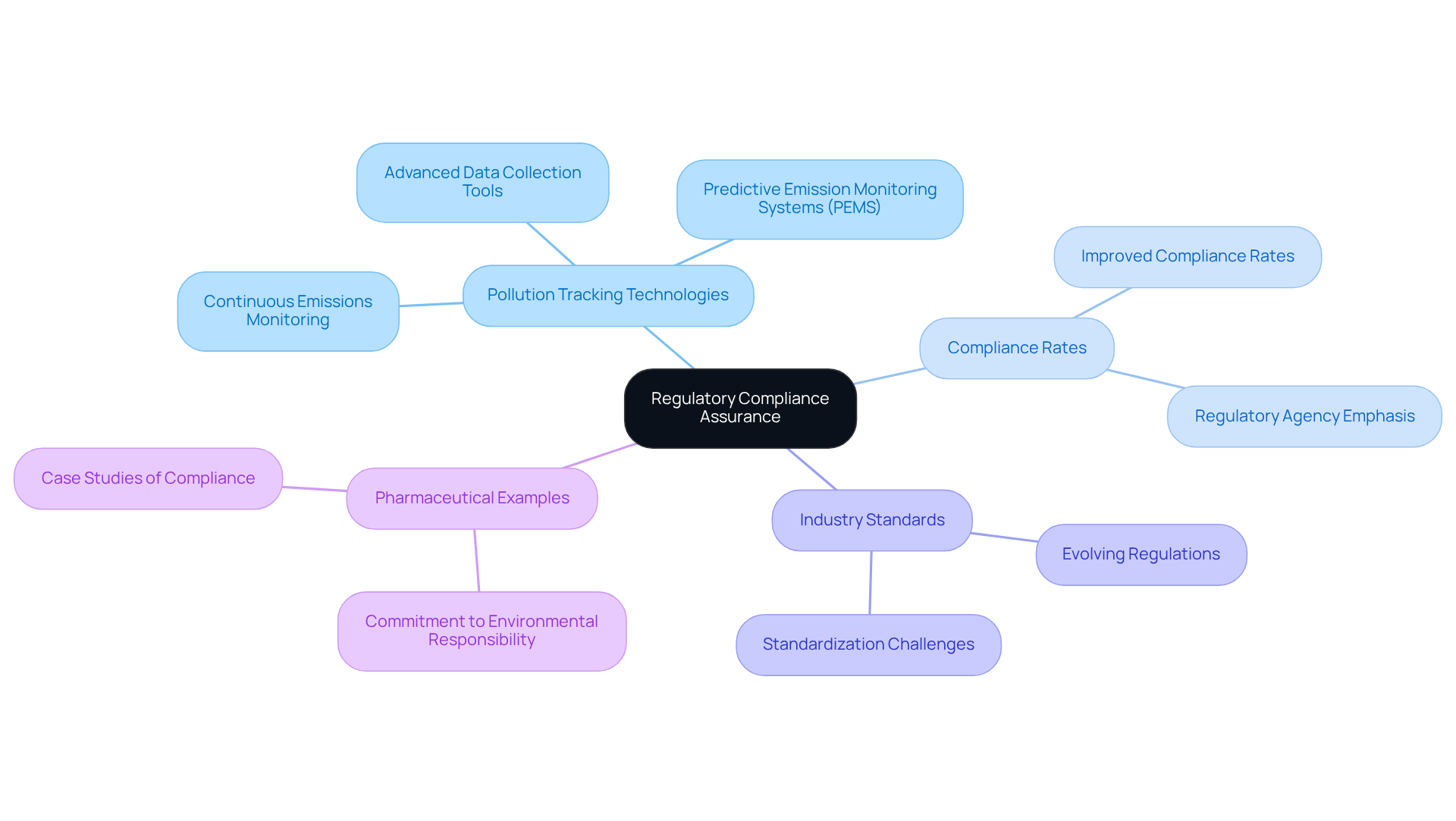
Regulatory Compliance Assurance: Meeting Industry Standards with Continuous Monitoring
Ongoing pollution tracking technologies are crucial for ensuring that organizations adhere to industry standards for discharges. These systems provide consistent and precise data, enabling companies to with regulations such as the Clean Air Act and other environmental laws. In 2023, the environmental monitoring sector accounted for a significant 78% of revenue in the pollution tracking market, underscoring its importance within regulatory frameworks.
For example, many pharmaceutical companies have effectively employed continuous emissions monitoring to fulfill Clean Air Act requirements, illustrating their commitment to environmental responsibility. Regulatory agencies emphasize the necessity of reliable discharge information, with environmental overseers asserting that regular monitoring is vital for effective compliance and safeguarding the environment.
Current regulations governing pollutant monitoring in the pharmaceutical sector mandate strict adherence to established standards, ensuring that discharges are accurately tracked and reported. Recent updates indicate that compliance rates with continuous emissions monitoring have markedly improved, reflecting an increasing acknowledgment of the need for robust monitoring solutions.
Industry standards for pollutant monitoring in pharmaceuticals are evolving, with a heightened focus on integrating advanced technologies to enhance data accuracy and reporting efficiency. As organizations adopt these frameworks, they not only fulfill compliance obligations but also cultivate a culture of accountability and transparency, ultimately bolstering their reputation within the industry.
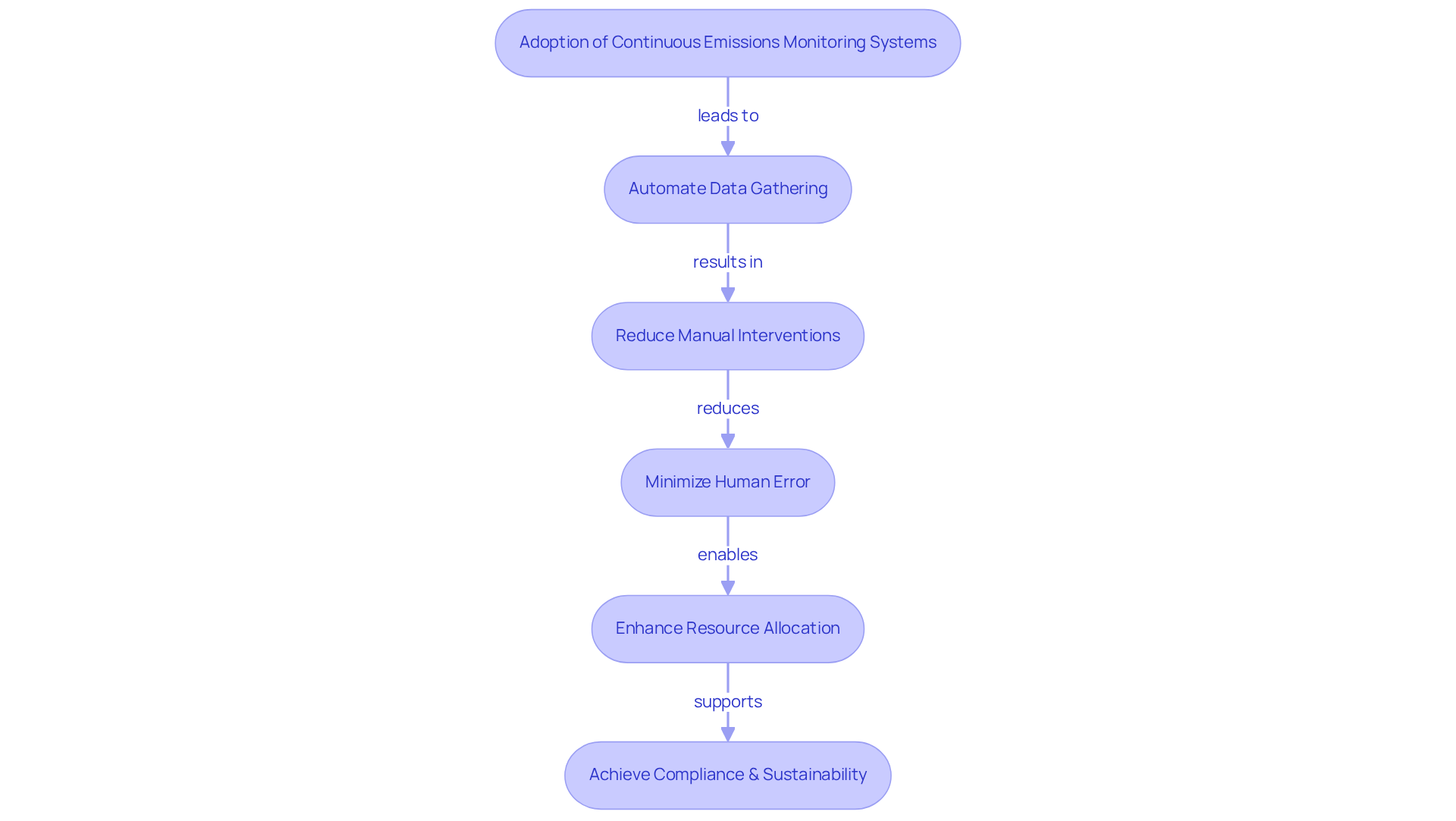
Operational Efficiency: Streamlining Processes with Continuous Emissions Monitoring
The adoption of continuous emissions monitoring systems significantly enhances operational efficiency by automating data gathering and reporting procedures. This automation reduces reliance on manual interventions, thereby minimizing human error and enabling organizations to allocate resources to more critical tasks. For instance, Emerson Electric Co.'s Rosemount XE10 continuous emissions monitoring system, introduced in 2021, has been pivotal in helping industrial plants comply with stringent environmental regulations while addressing sustainability demands. By streamlining emissions data collection, pharmaceutical companies can concentrate on core operations, ensuring adherence to environmental regulations and ultimately achieving enhanced productivity and cost savings.
Industry specialists underscore the transformative effect of automation on regulatory processes. As noted by a prominent expert in the field, the incorporation of automated systems not only streamlines reporting but also enhances the overall regulatory framework within organizations. This shift towards automation is evident in the increasing adoption of and continuous emissions monitoring across various sectors, including pharmaceuticals, where the demand for precise and prompt emissions data is paramount.
Moreover, the successful installation of over 673 continuous emissions monitoring units in the pharmaceutical sector underscores the industry's commitment to regulatory adherence and environmental responsibility. These systems facilitate streamlined processes, enabling companies to uphold high standards of quality while navigating complex regulatory landscapes. The outcome is a more efficient operational model that supports both compliance and sustainability objectives.
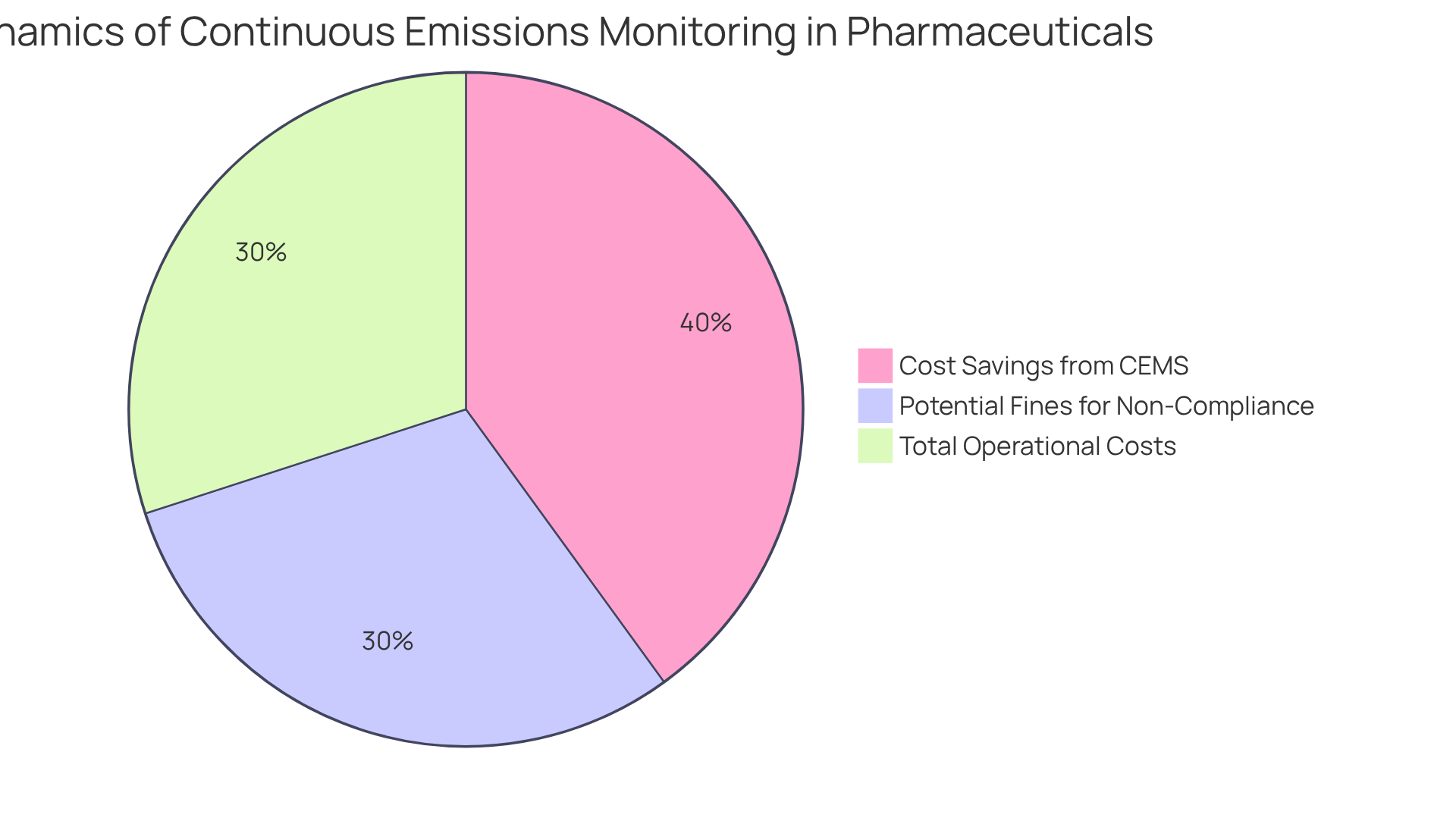
Cost Savings: Reducing Operational Expenses through Continuous Emissions Monitoring
Continuous emissions monitoring provides substantial cost-saving opportunities for organizations within the pharmaceutical sector. By diminishing reliance on manual pollution assessments and reducing the risk of non-compliance fines, businesses can effectively lower their operational costs. For instance, the average fine for exceeding carbon output can escalate to $150 per ton, underscoring the financial implications of compliance.
Furthermore, continuous emissions monitoring (CEMS) provides real-time data that helps identify inefficiencies in operational processes, enabling organizations to optimize resource utilization and achieve additional cost savings. Financial analysts note that adopting this system for continuous emissions monitoring can lead to a significant reduction in overall operational expenses, with projections suggesting decreases of up to 40% in anticipated total costs related to carbon output.
This proactive approach not only but also contributes to the development of a more sustainable and economically viable operational model in the pharmaceutical sector.
Environmental Sustainability: Promoting Cleaner Operations with Continuous Monitoring
Ongoing monitoring of pollutants is essential for fostering environmental sustainability within the pharmaceutical sector. By delivering real-time information on pollutants, these solutions empower organizations to identify and address their environmental impacts effectively, ensuring compliance with stringent regulations. This proactive approach not only facilitates adherence to legal standards but also strengthens , enhancing the organization’s reputation as a responsible corporate citizen.
The market for pollutant monitoring systems is projected to grow significantly, reaching USD 4.96 billion by 2030, driven by increasing regulatory demands and a shift towards sustainable practices. Companies that implement environmental management systems can demonstrate their commitment to reducing pollutants, which is increasingly recognized as a vital aspect of corporate accountability.
For instance, leading pharmaceutical firms are adopting continuous emissions monitoring to promote cleaner operations and reduce their carbon footprints. This aligns with the perspectives of sustainability leaders who assert that effective emissions monitoring is crucial for achieving corporate sustainability objectives. As industry experts note, "Emission Monitoring Systems (EMS) are essential tools for measuring and reporting pollutant concentrations, supporting both regulatory compliance and operational optimization."
Moreover, organizations that leverage such systems not only enhance their compliance posture but also gain a competitive edge by showcasing their dedication to environmental stewardship. This commitment is reflected in the growing trend of companies integrating pollution data with process-control software, which fosters improved decision-making and operational efficiency. As the demand for cleaner operations escalates, the role of continuous emissions monitoring systems will become increasingly pivotal in shaping sustainable practices across the pharmaceutical industry.
Enhanced Safety: Protecting Worker Health with Continuous Emissions Monitoring
Continuous emissions monitoring is essential for enhancing workplace safety by delivering real-time information on hazardous discharges. By consistently tracking discharge levels, organizations can swiftly identify potential health risks and implement immediate corrective actions to safeguard their workforce. This proactive approach not only ensures compliance with occupational health and safety regulations but also fosters a within the organization. Consequently, companies witness improved employee morale and productivity, reinforcing the principle that a safe work environment is crucial for operational success.
Experts assert that monitoring hazardous emissions is vital for protecting worker health, especially in the pharmaceutical sector, where exposure to harmful substances can have severe consequences. Organizations that effectively leverage such systems demonstrate a commitment to employee well-being, ultimately resulting in a healthier, more engaged workforce.
A transformative case study involving AVS Life Sciences exemplifies this dedication: the company successfully assisted a leading biotechnology firm in upgrading their GMP facility by implementing advanced emission monitoring systems. This upgrade not only enhanced the facility's operational capabilities but also underscored the significance of rigorous quality control processes, ultimately contributing to a safer work environment.
By employing the system for continuous emissions monitoring, the facility was able to ensure compliance with safety regulations and significantly reduce hazardous emissions. Successful examples of organizations utilizing these systems include those that have reported substantial improvements in workplace safety, showcasing the effectiveness of such systems in protecting employee health.
Scalability: Adapting Continuous Emissions Monitoring to Business Growth
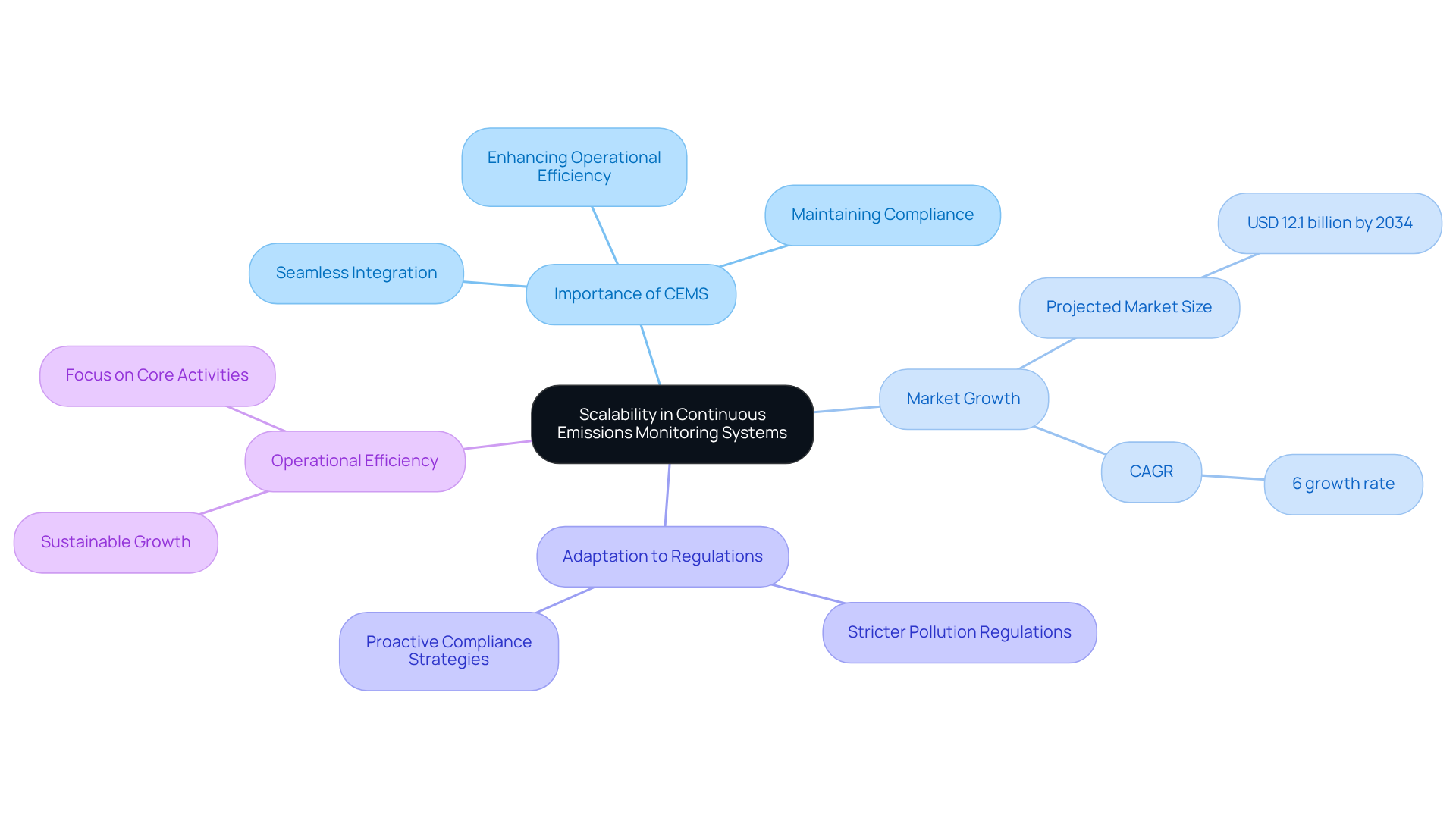
Continuous emissions monitoring is crucial for pharmaceutical firms in the midst of expansion due to the considerable scalability provided by Continuous Emissions Monitoring Systems (CEMS). As organizations grow their operations, these management systems can be seamlessly integrated to accommodate new processes and evolving regulatory demands. This adaptability is crucial for maintaining compliance without disrupting existing workflows. Notably, the worldwide monitoring network market is anticipated to reach USD 12.1 billion by 2034, with the demand for CEMS expected to increase at a compound annual growth rate (CAGR) of 6%. This growth underscores the necessity for pharmaceutical companies to implement robust monitoring systems that can evolve alongside their business needs.
Moreover, industry leaders emphasize the critical importance of adapting continuous emissions monitoring to meet new regulatory requirements. As more stringent pollution regulations are enacted, companies must ensure their continuous emissions monitoring systems are equipped to handle heightened scrutiny. This proactive strategy not only facilitates adherence but also , enabling firms to focus on their core activities while fulfilling their environmental responsibilities. The ability to effectively expand systems positions pharmaceutical firms to thrive in a competitive landscape, ultimately fostering sustainable growth and compliance.
Reliability: Ensuring Consistent Performance with Continuous Emissions Monitoring
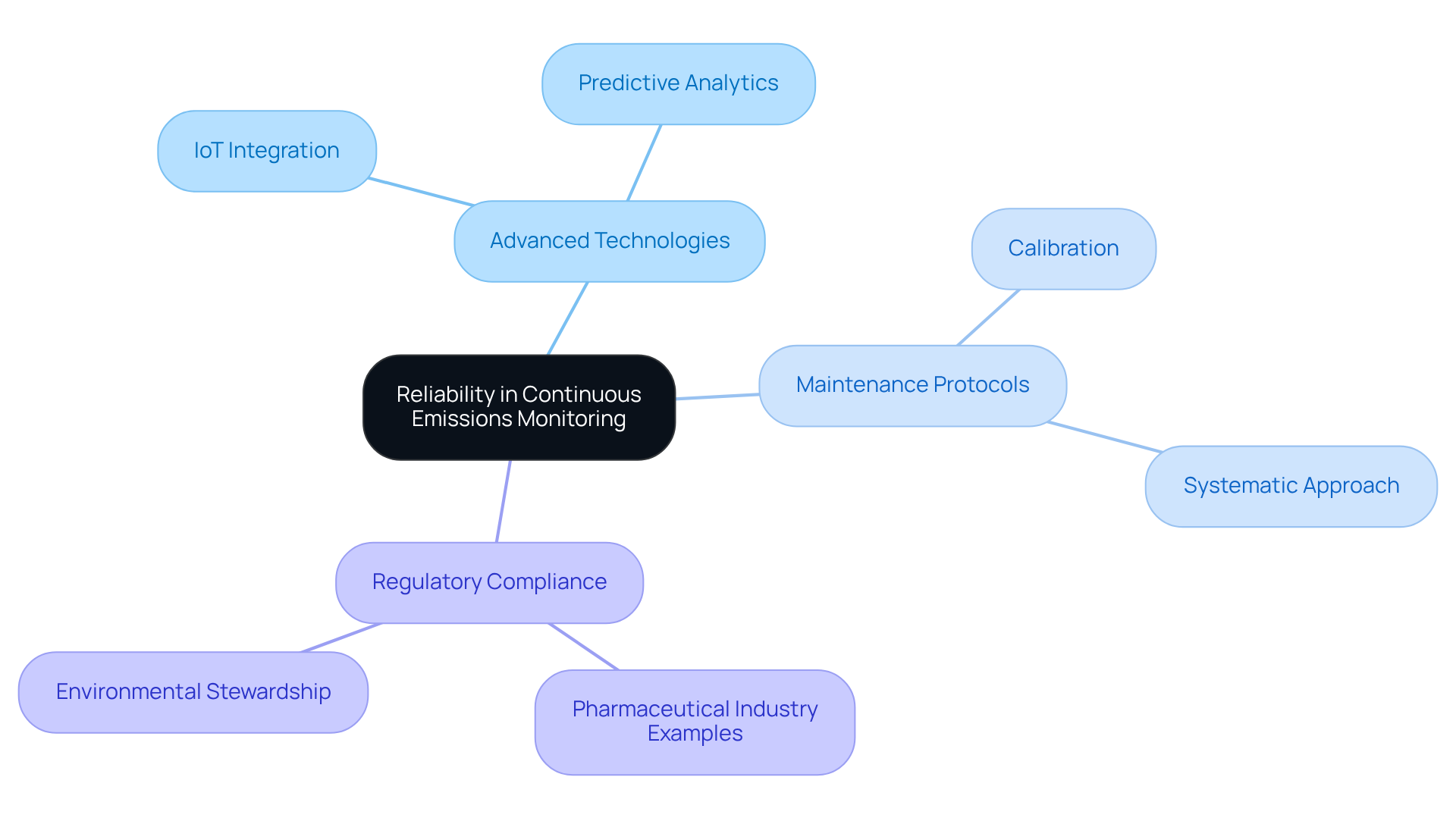
Continuous emissions monitoring solutions (CEMS) are designed for reliability, ensuring consistent performance in tracking pollutant levels. By harnessing advanced technologies such as IoT integration and predictive analytics, alongside rigorous maintenance protocols, organizations can rely on their monitoring systems to provide accurate and timely data. This reliability is crucial for meeting regulatory standards and making informed decisions regarding environmental management.
Industry experts emphasize that a systematic approach to maintenance and calibration is vital for sustaining operational efficiency and compliance. For instance, pharmaceutical firms that have implemented CEMS have reported significant improvements in tracking accuracy, enabling them to adhere to stringent environmental regulations effectively.
The integration of cutting-edge technology not only bolsters the reliability of continuous emissions monitoring but also facilitates proactive management strategies, ultimately nurturing a culture of environmental stewardship within the industry.
As your trusted partner in quality management and regulatory compliance, AVS Life Sciences empowers organizations to maintain alignment with regulatory standards. With AVS's , you can be assured of expert guidance in navigating the complexities of environmental regulations.
Ease of Use: Simplifying Compliance Management with Continuous Emissions Monitoring
Ongoing monitoring solutions for pollutants are crucial in addressing the complexities of regulatory management for pharmaceutical companies. These systems emphasize ease of use, significantly streamlining the compliance process. With intuitive interfaces and , users can effortlessly access and interpret emissions data. This streamlined method reduces the intricacies associated with regulatory tasks, allowing organizations to focus on maintaining high standards with minimal effort. Notably, enterprises employing continuous emissions monitoring have reported enhanced operational efficiency and improved compliance with regulations. This illustrates the vital role these tools play in navigating the intricate landscape of environmental laws. Compliance managers have observed that the simplicity of these systems not only facilitates smoother data management but also nurtures a proactive compliance culture within their organizations, fostering an environment of accountability and excellence.
Conclusion
Continuous emissions monitoring systems (CEMS) are indispensable for ensuring compliance within the pharmaceutical industry. By delivering real-time data and enabling proactive decision-making, these systems empower organizations to meet stringent regulatory requirements while simultaneously enhancing operational efficiency. The integration of CEMS not only supports adherence to environmental standards but also cultivates a culture of accountability and sustainability.
Key benefits of continuous emissions monitoring are multifaceted, as highlighted throughout the article. These systems improve safety by tracking hazardous discharges and deliver significant cost savings through optimized resource utilization. Furthermore, their scalability allows organizations to adapt seamlessly to growth and evolving regulations, ensuring ongoing compliance and operational excellence.
As the pharmaceutical sector evolves, the significance of continuous emissions monitoring cannot be overstated. Organizations are urged to embrace these advanced systems not only for regulatory compliance but also as a strategic investment in their operational future. By prioritizing environmental stewardship and leveraging real-time monitoring technologies, companies can lead the way in creating safer workplaces, fostering sustainability, and achieving long-term success.
Frequently Asked Questions
What services does AVS Life Sciences provide for regulatory compliance in the pharmaceutical sector?
AVS Life Sciences offers comprehensive services including validation, quality assurance consulting, and engineering support tailored to ensure adherence to environmental regulations within the pharmaceutical sector.
How does AVS Life Sciences assist with continuous emissions monitoring?
AVS Life Sciences facilitates the implementation of effective continuous emissions monitoring systems that comply with stringent regulatory requirements, ensuring accurate and efficient monitoring of discharges.
What recent changes in regulations have impacted emissions monitoring?
Recent regulations from the U.S. EPA have broadened reporting requirements to include small and mid-sized manufacturing facilities, increasing the emphasis on emissions monitoring.
What is the projected market growth for continuous emissions monitoring systems?
The market for continuous emissions monitoring systems is projected to reach USD 8.5 billion by 2033.
Why is real-time data collection important in pharmaceutical compliance?
Real-time data collection allows organizations to swiftly detect deviations from regulatory standards, implement corrective measures, and enhance operational efficiency, making decision-making processes 29% quicker.
How do continuous emissions monitoring systems utilize technology?
These systems use AI and machine learning to analyze real-time data, facilitating early identification of safety concerns and optimizing regulatory reporting.
What role does ongoing pollution tracking play in regulatory compliance?
Ongoing pollution tracking technologies provide consistent and precise data necessary for organizations to demonstrate compliance with regulations like the Clean Air Act.
What percentage of revenue in the pollution tracking market does environmental monitoring account for?
In 2023, the environmental monitoring sector accounted for 78% of revenue in the pollution tracking market.
How have compliance rates with continuous emissions monitoring changed recently?
Compliance rates with continuous emissions monitoring have markedly improved, reflecting a growing acknowledgment of the need for robust monitoring solutions.
What is the overall impact of integrating advanced technologies in pollutant monitoring?
The integration of advanced technologies enhances data accuracy and reporting efficiency, helping organizations fulfill compliance obligations while fostering a culture of accountability and transparency.
