10 Benefits of Automated Inspection for Pharmaceutical Compliance

Overview
The article discusses the significant advantages of automated inspection systems within the pharmaceutical industry, emphasizing their critical role in enhancing compliance, operational efficiency, and product quality. In light of the compliance challenges faced by the industry, integrating advanced technologies such as AI and machine learning emerges as a robust solution. These systems not only significantly reduce human error but also streamline processes and ensure adherence to regulatory standards. Ultimately, this integration contributes to the production of safer pharmaceuticals and improved operational outcomes. By adopting these automated solutions, organizations can navigate the complexities of compliance with greater ease, paving the way for enhanced productivity and reliability in their operations.
Introduction
The pharmaceutical industry faces relentless pressure to comply with stringent standards while ensuring the quality and safety of its products. In response, automated inspection systems have emerged as a transformative solution, delivering a myriad of benefits that not only streamline operations but also bolster adherence to regulatory requirements. As organizations increasingly embrace these advanced technologies, critical questions arise regarding their true impact on operational efficiency and quality assurance.
What specific advantages do automated inspection systems offer, and how can they empower pharmaceutical companies to adeptly navigate the complexities of compliance in an ever-evolving landscape?
AVS Life Sciences: Tailored Automated Inspection Solutions for Pharmaceutical Compliance
AVS Life Sciences excels in providing tailored automated inspection solutions specifically designed for the pharmaceutical sector. By leveraging advanced technologies, AVS empowers clients to meet stringent regulatory standards, including GXP and FDA guidelines, while significantly enhancing product excellence. This customized approach facilitates the seamless integration of that enhance review processes and elevate operational efficiency, thereby reducing the risk of non-compliance. Furthermore, adherence to Standard Operating Procedures (SOPs) and the management of Data Integrity Deviations are critical components of our operational practices.
The pharmaceutical evaluation machines market is projected to grow from USD 862.0 million in 2023 to USD 1.62 billion by 2030, propelled by the rising adoption of advanced assessment technologies. In 2023, automated inspection machines accounted for 76.0% of the market share, underscoring their vital role in maintaining standards and compliance. Additionally, the integration of cutting-edge technologies, such as AI and machine learning, is revolutionizing evaluation capabilities, enabling real-time defect detection and enhancing overall efficiency.
Successful implementations of automated inspection technologies in leading pharmaceutical companies have demonstrated substantial improvements in quality assurance processes. These advancements not only streamline operations but also play a crucial role in upholding high standards of safety and efficacy in pharmaceutical products, ultimately fostering increased trust among consumers and regulatory bodies alike. As emphasized by industry authorities, 'Regulatory adherence is essential, with strict demands from organizations like the FDA and EMA,' highlighting the importance of automated inspection solutions in navigating complex regulatory landscapes.

Enhanced Regulatory Compliance Through Automated Inspection Systems
Automated inspection frameworks are pivotal in enhancing regulatory compliance, ensuring strict adherence to Good Manufacturing Practices (GMP) and other essential industry standards. These sophisticated systems facilitate real-time oversight and comprehensive documentation of evaluation procedures, which are critical for audits and regulatory reviews. By integrating automated inspection, pharmaceutical firms can significantly minimize human error, guaranteeing that each product meets the necessary specifications prior to market launch.
Recent research indicates that approximately 60% of pharmaceutical firms have adopted automated processes to bolster their GMP adherence initiatives. This transformation not only streamlines operations but also fosters a culture of excellence, as provide consistent and impartial evaluations of manufacturing processes.
Furthermore, organizations utilizing these systems report improved adherence outcomes, with many experiencing a reduction in audit findings and enhanced overall product quality. As the industry evolves, the integration of automated inspection technologies will be vital in maintaining high standards of regulatory compliance and ensuring the safety and efficacy of pharmaceutical products.
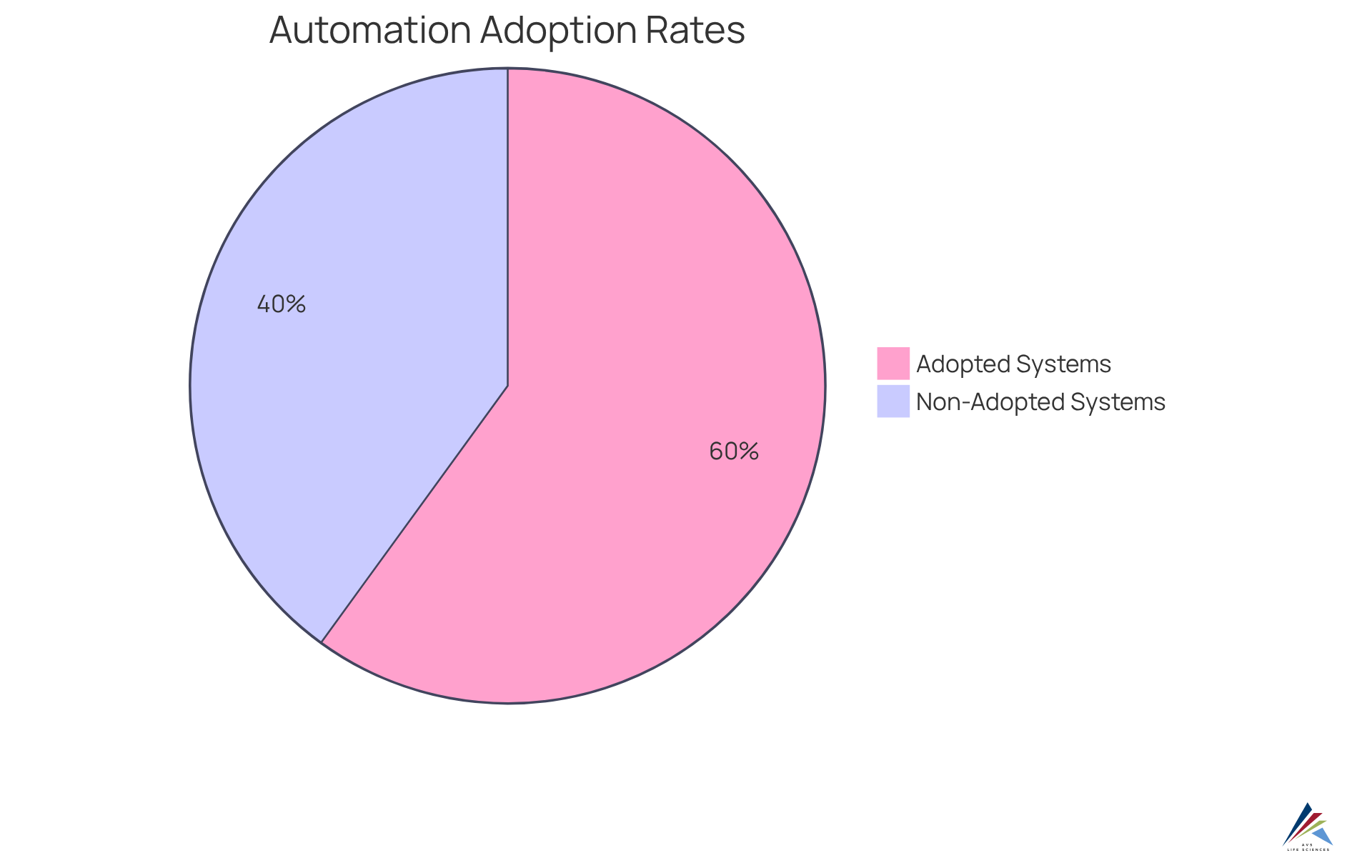
Increased Operational Efficiency with Automated Inspection Technologies
The application of automated inspection technologies significantly enhances operational efficiency in pharmaceutical production, addressing critical compliance challenges by drastically reducing assessment times and minimizing downtime. Automated systems excel at processing vast quantities of products with remarkable speed and precision, enabling manufacturers to achieve high throughput rates without compromising quality.
For instance, automation can accelerate evaluation processes by up to 100 times, resulting in a staggering 90% decrease in reporting mistakes. This efficiency not only but also empowers companies to swiftly adapt to evolving market demands. Consequently, organizations can sustain a competitive advantage while ensuring strict adherence to regulatory standards.
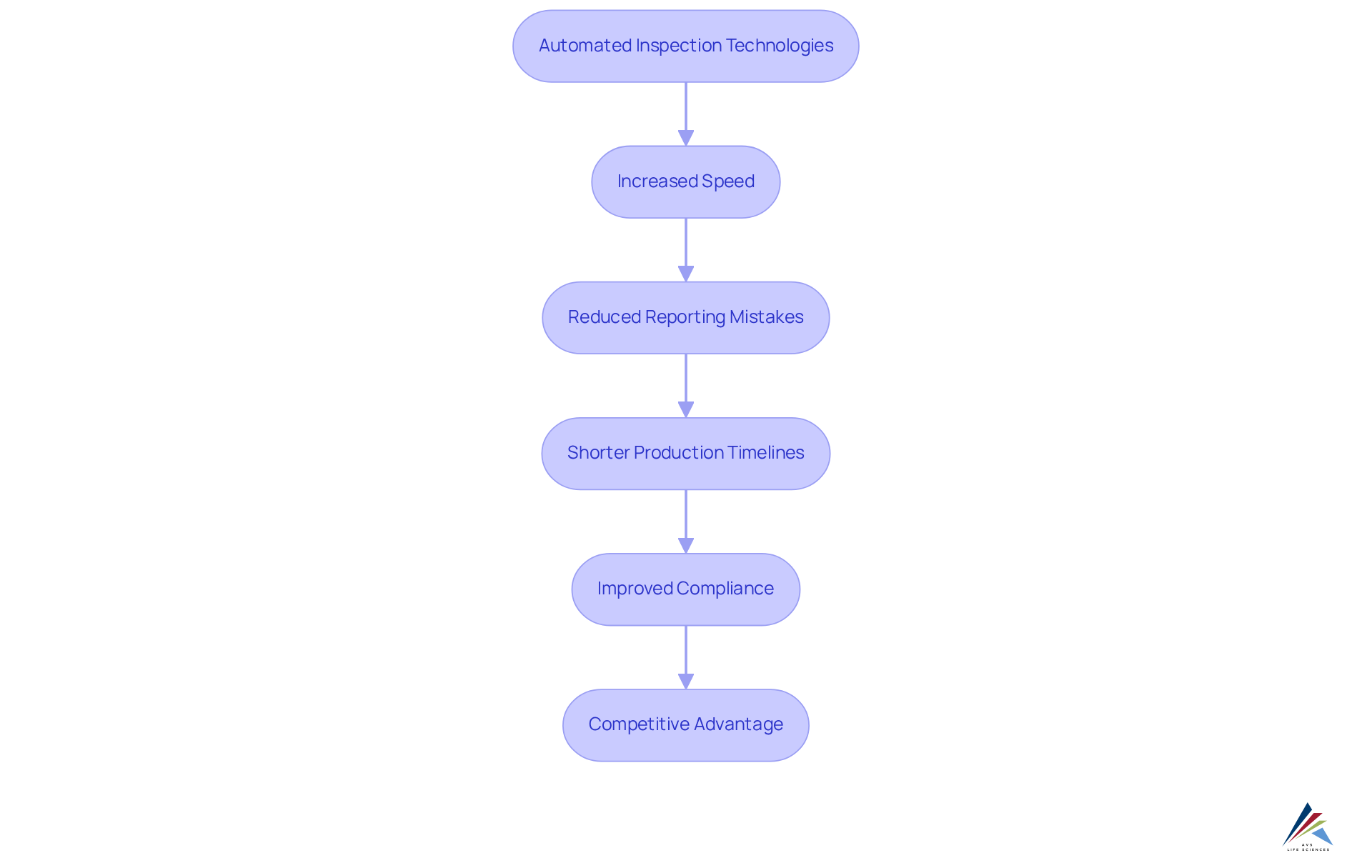
Furthermore, the incorporation of machine vision technology exemplifies this transformation, facilitating thorough evaluations of pill uniformity and guaranteeing that only flawless products reach consumers. Overall, the transition towards automated inspection in evaluations is set to redefine assurance standards in the pharmaceutical sector, thereby enhancing both productivity and compliance.
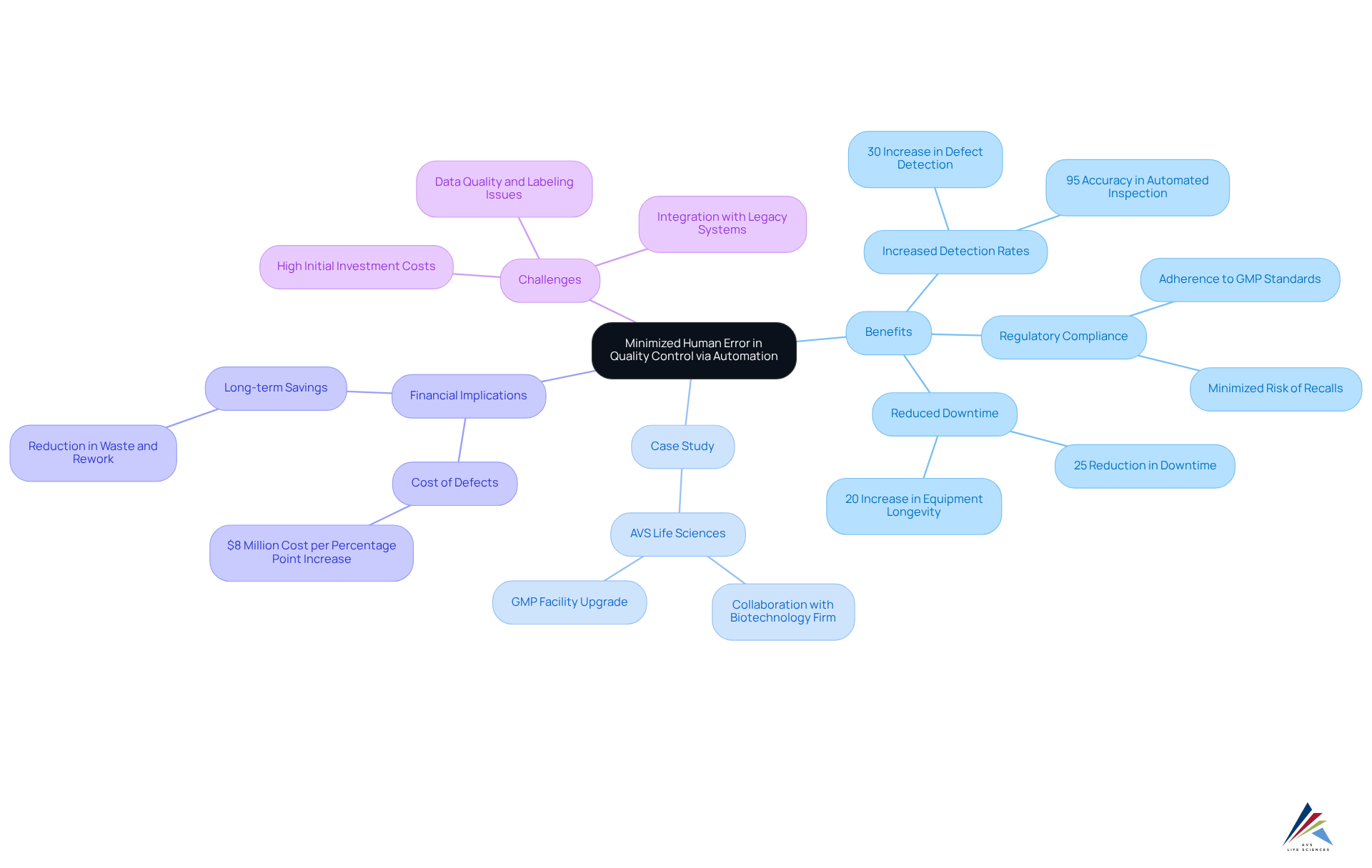
Minimized Human Error in Quality Control via Automation
Automated evaluation mechanisms significantly mitigate human errors, a critical factor in maintaining high-quality standards in pharmaceutical production. A transformative case study involving AVS Life Sciences exemplifies this point, where the company adeptly assisted a leading biotechnology firm in upgrading their GMP facility. This enhancement not only refined the assurance processes but also ensured strict adherence to regulatory standards, underscoring AVS Life Sciences' commitment to excellence in the life sciences sector.
By leveraging advanced algorithms and machine learning, these systems can identify defects and inconsistencies that human inspectors may overlook. For instance, organizations employing AI-driven tools have reported a 30% increase in defect detection rates, demonstrating the technology's efficacy in pinpointing flaws early in the production process.
The precision of automated inspection not only elevates product quality but also guarantees compliance with regulatory standards. This consistency is crucial in minimizing the risk of costly recalls and regulatory penalties, which can profoundly affect a company's reputation and financial status. Indeed, a one percentage point increase in defect rates can incur costs of up to $8 million annually for an automotive facility, highlighting the financial ramifications of control failures.
Moreover, automated inspection mechanisms operate continuously, delivering detection rates that often exceed 95%. This capability enables manufacturers to maintain high throughput while ensuring that each product meets performance standards. Consequently, firms that have integrated automated evaluation systems report a 25% reduction in downtime and a 20% enhancement in equipment longevity, empowering them to meet stringent deadlines without compromising standards.
During the upgrade process, the biotechnology client encountered challenges, such as anomalies in test results stemming from improperly installed barcode scanner cameras. This oversight necessitated a thorough review of their control processes, resulting in that bolstered their operational efficiency.
In conclusion, the incorporation of machine learning and automated inspection in assessment processes not only enhances defect detection rates but also strengthens compliance efforts, ultimately fostering improved operational efficiency and reducing the risk of errors in pharmaceutical manufacturing. Businesses are encouraged to explore AI-powered evaluation frameworks to enhance their quality assurance procedures.
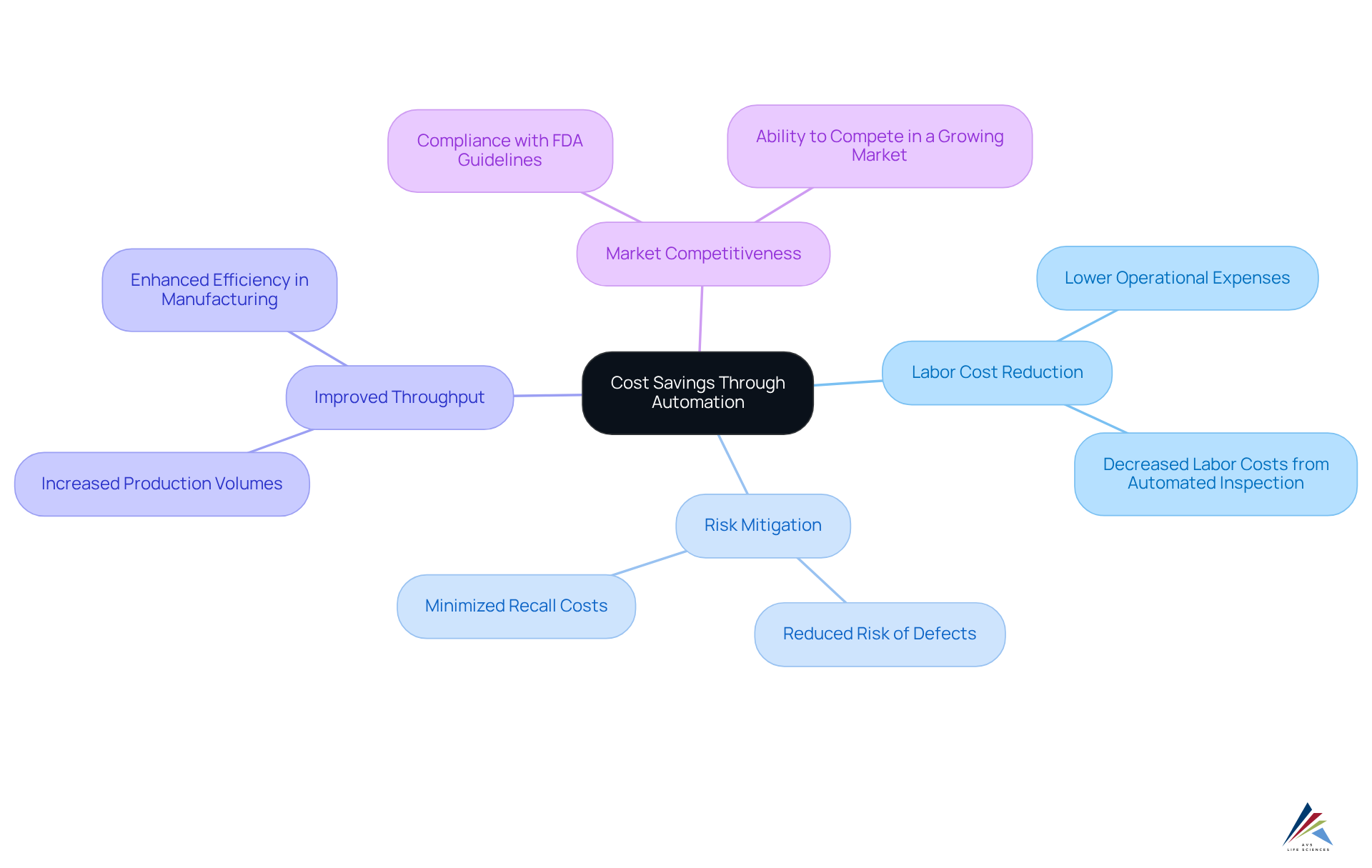
Cost Savings Achieved Through Automated Inspection Implementation
Introducing automated evaluation frameworks presents a compelling opportunity for pharmaceutical firms to achieve significant cost reductions. A prime example is AVS Life Sciences' successful enhancement of a biotechnology GMP facility, which illustrates the transformative impact of automation.
By decreasing labor costs associated with automated inspection, organizations can substantially lower their operational expenses. Although specific statistics on labor cost reduction were not provided, it is widely acknowledged that automation fosters lower costs and enhanced efficiency.
Furthermore, these automated inspection solutions mitigate the risk of defects, which can lead to costly recalls and damage to reputation. An additional advantage is improved throughput; automated processes facilitate increased production volumes without a corresponding rise in expenses. This efficiency not only enhances productivity but also contributes to a more robust bottom line, making automation a for the industry.
Given that the pharmaceutical market has expanded at an annual rate of 5.8% since 2017, the importance of implementing automation to maintain competitiveness and comply with FDA guidelines and Good Manufacturing Practices (GMP) cannot be overstated. The integration of digitized batch processing solutions by AVS Life Sciences exemplifies how such advancements, particularly automated inspection, can optimize vaccine manufacturing efficiency, further solidifying the case for automation in this sector.
Real-Time Data Analysis for Informed Compliance Decisions
Automated inspection is revolutionizing decision-making in the pharmaceutical sector through real-time data analysis. This capability enables companies to swiftly identify deviations from established standards, facilitating . The importance of such prompt actions is paramount, as they are crucial for maintaining GXP and FDA regulations, ensuring product quality, and adhering to stringent Standard Operating Procedures (SOPs).
By leveraging real-time insights, pharmaceutical firms can enhance their compliance frameworks, aligning with regulatory expectations while minimizing the risk of costly breaches. The integration of these technologies not only enhances operational efficiency but also fosters a culture of proactive regulatory management, ultimately leading to improved product quality, patient safety, and the implementation of automated inspection.
AVS Life Sciences is at the forefront of these advancements, providing expert solutions in GMP compliance, validation, and engineering specifically designed for the pharmaceutical and biotechnology industries.
Scalable Automated Inspection Solutions for Growing Pharmaceutical Needs
Automated inspection solutions are engineered for scalability, empowering pharmaceutical companies to seamlessly adapt to shifting production demands. As organizations expand, they can enhance their evaluation capabilities without extensive reconfiguration or additional resources. This inherent adaptability is crucial, particularly as India produces approximately 60,000 generic medications and over 400 bulk substances, necessitating robust monitoring systems to ensure compliance with .
For example, Maharashtra, which represents 29% of India's manufacturing units, is significantly scaling its drug testing capacity from 8,000 to 80,000 samples per year, reflecting the pressing need for flexible inspection solutions.
AVS Life Sciences exemplifies this commitment to excellence in management and regulatory compliance, as demonstrated in a transformative case study where they assisted a leading biotechnology company in upgrading their GMP facility. During this upgrade, challenges such as anomalies in test results due to improperly installed barcode scanner cameras were identified, leading to critical lessons learned regarding quality assurance processes.
By investing in automation, companies can uphold high-quality standards through automated inspection while efficiently addressing increased production demands, positioning it as a vital component for sustainable growth in the competitive pharmaceutical landscape.
AVS Life Sciences' expertise ensures that these automated inspection solutions not only enhance evaluation capabilities but also adhere to stringent regulatory standards, ultimately supporting the industry's goal of providing safe and effective medicines.
AI-Driven Defect Detection in Automated Inspection Systems
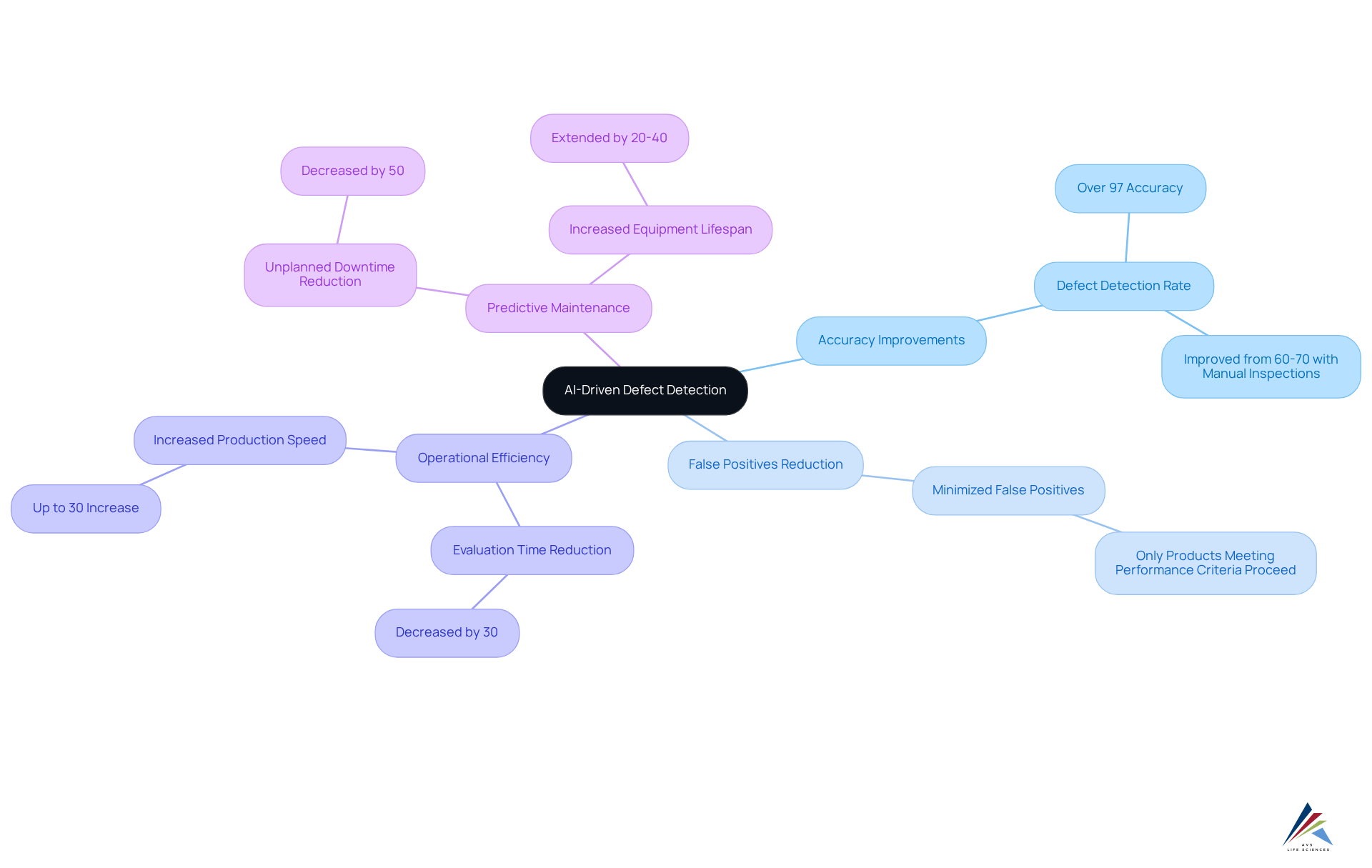
AI-powered flaw identification is revolutionizing automated inspection processes in the pharmaceutical sector. By leveraging machine learning algorithms, these systems analyze historical data to identify patterns and anomalies with remarkable precision. This capability considerably improves , attaining accuracy levels surpassing 97%, in contrast to the 60-70% commonly observed with manual evaluations.
Furthermore, the incorporation of AI minimizes false positives, guaranteeing that only products that satisfy strict performance criteria proceed through the manufacturing process. Producers can anticipate better adherence to regulatory standards, such as GXP and FDA rules, along with increased operational efficiency, with evaluation durations decreased by as much as 30%.
The impact of machine learning extends beyond defect detection; it also facilitates predictive maintenance, which can decrease unplanned downtime by as much as 50%, thereby optimizing production workflows. Overall, the implementation of AI in automated inspection frameworks is a game-changer for the pharmaceutical sector, elevating assurance to new heights.
AVS Life Sciences stands at the forefront of this transformation, providing comprehensive quality management and regulatory compliance solutions tailored for the life sciences sector, including the implementation of standard operating procedures (SOPs) and ensuring data integrity.
Training and Skill Development for Effective Automated Inspection Use
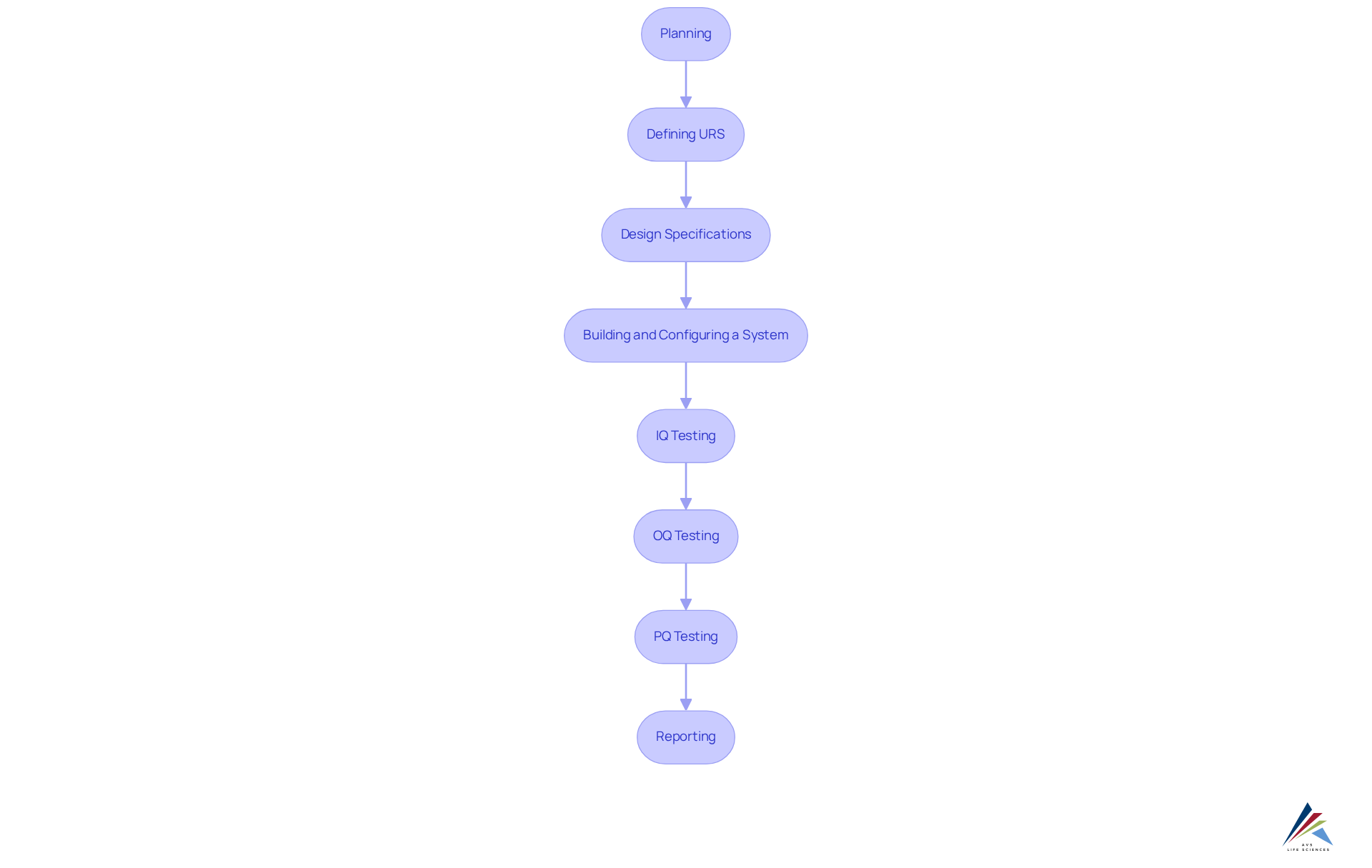
To fully leverage the advantages of automated inspection, it is imperative to implement comprehensive training and skill development programs. Employees must be equipped with the knowledge and skills necessary to operate these advanced technologies effectively, particularly within the context of the computer validation process. This process encompasses critical stages, including:
- Planning: Management determines the necessary budget and timeline for computer system validation.
- Defining URS (User Requirement Specifications): This outlines the tasks that must be performed.
- Design Specifications: The team delineates the functionality and operation of the solution.
- Building and Configuring a System: Development of configured scripts for software design takes place here.
- IQ (Installation Qualification) Testing: Testing is conducted to ensure proper installation methods.
- OQ (Operational Qualification) Testing: This stage verifies that the system operates as intended.
- PQ (Performance Qualification) Testing: Simulating worst-case scenarios ensures continued functionality.
- Reporting: Documenting all actions taken during testing phases is crucial.
Understanding these stages is essential for ensuring . Continuous training guarantees that personnel remain informed about the latest developments in evaluation technology and validation standards, fostering a culture of ongoing enhancement and compliance within the organization. Furthermore, partnering and collaborating with seasoned colleagues at AVS Life Sciences significantly enhances personal and professional development, positioning it as an ideal environment for career advancement.
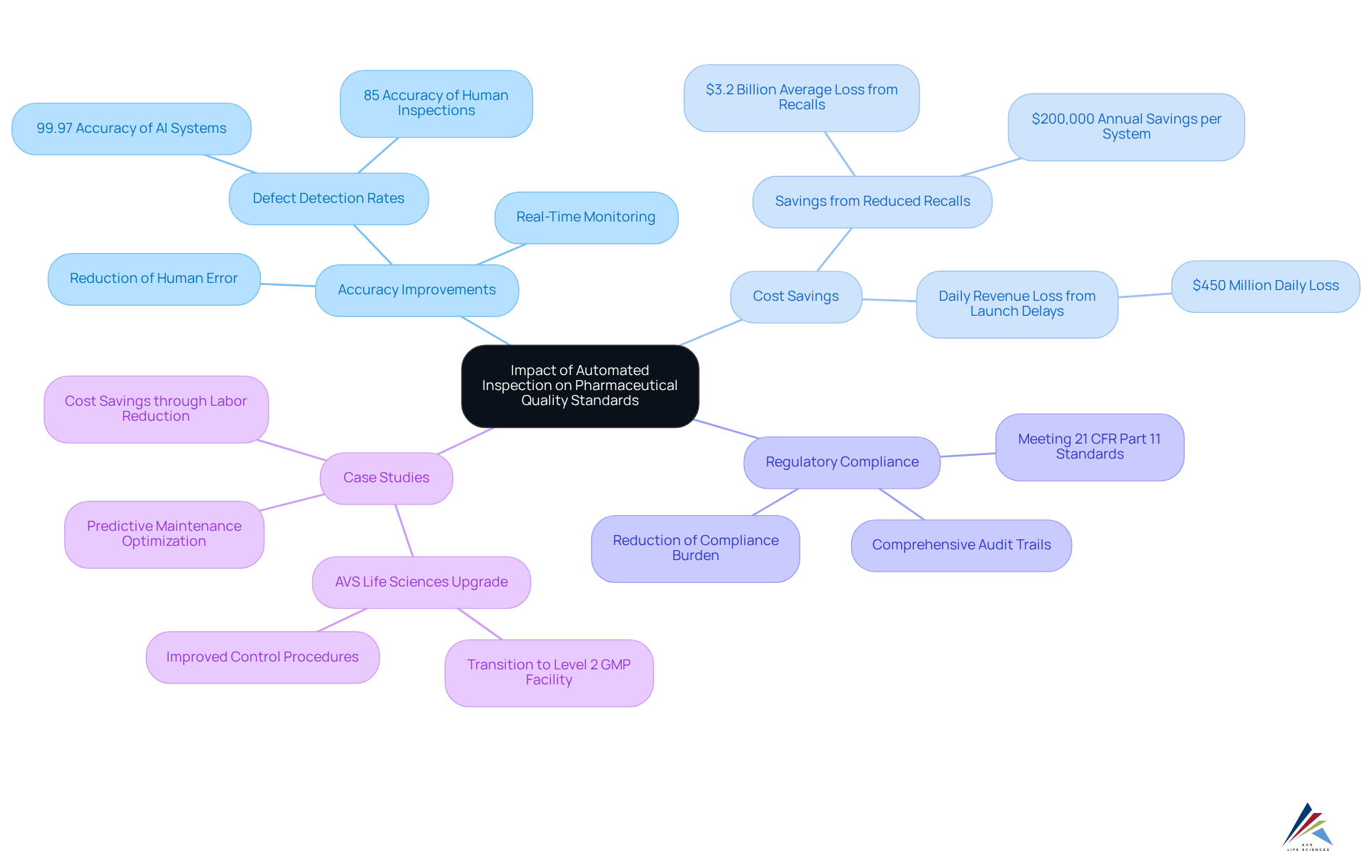
Overall Impact of Automated Inspection on Pharmaceutical Quality Standards
Automated evaluation profoundly influences pharmaceutical quality standards by significantly enhancing accuracy, efficiency, and compliance. These systems streamline production processes and ensure that products meet stringent regulatory requirements, ultimately contributing to safer and higher-quality pharmaceuticals. For instance, automated inspection technologies can achieve defect detection rates of up to 99.97%, which far surpasses the 85% accuracy of inspections conducted by humans. This remarkable precision decreases the chances of defects, which can lead to in revenue loss during the first year of a major drug recall.
Moreover, the integration of automated systems allows for real-time monitoring and documentation, creating comprehensive audit trails that satisfy regulatory bodies without the burden of manual documentation. This capability is vital, as 87% of launch delays in pharmaceuticals arise from avoidable issues, leading to daily revenue losses of $450 million for postponed billion-dollar drug launches.
A transformative case study exemplifying this impact is AVS Life Sciences' successful upgrade of a biotechnology GMP facility for a leading San Francisco-based biotechnology company. AVS facilitated the transition from a Biosafety Level 1 GMP facility to a Level 2 GMP facility, ensuring adherence to assurance standards. This upgrade enabled the client to produce medication using lentivirus vector material while improving their control procedures. During this process, important lessons were learned, particularly regarding anomalies in test results due to improperly installed barcode scanner cameras. This partnership not only enhanced operational efficiency but also allowed the client to concentrate on creating medicines that enhance patient well-being.
Instances of enhanced quality management through automated inspection methods are evident in numerous case studies. Producers have reported savings of up to $200,000 per unit each year by decreasing dependence on human inspectors and reallocating staff to more strategic roles. Furthermore, the adoption of AI visual evaluation technologies has resulted in a defect rate of only 0.003% during launches, demonstrating a 99.8% enhancement compared to earlier attempts.
As the pharmaceutical evaluation machines market is projected to grow at a CAGR of 9.9% from 2024 to 2030, the shift towards automation signifies a commitment to excellence in quality management and regulatory compliance, ensuring that patient safety remains a top priority. AVS Life Sciences is positioned as a leader in this trend, highlighting their contribution to the advancement of automated inspection systems within the industry.
Conclusion
The integration of automated inspection systems in the pharmaceutical industry signifies a monumental advancement in achieving enhanced compliance, operational efficiency, and product quality. By adopting these cutting-edge technologies, pharmaceutical companies can adeptly navigate complex regulatory landscapes while ensuring their products consistently meet the highest standards of safety and efficacy.
Key insights from the article illuminate the multifaceted benefits of automated inspection, encompassing:
- Minimized human error
- Real-time data analysis
- Significant cost savings
The implementation of AI-driven defect detection further amplifies the precision of evaluations, drastically reducing the likelihood of costly recalls and reinforcing overall compliance. Additionally, the scalability of these solutions empowers organizations to adapt to increasing production demands without compromising quality.
As the pharmaceutical landscape continues to evolve, embracing automated inspection technologies is not merely an option; it is a necessity for companies striving to maintain a competitive edge. Investing in these systems, coupled with comprehensive training and skill development, will enable organizations to uphold rigorous quality standards and cultivate a culture of continuous improvement. The future of pharmaceutical compliance hinges on the strategic integration of automation—an essential step toward ensuring patient safety and operational excellence.
Frequently Asked Questions
What is AVS Life Sciences known for?
AVS Life Sciences specializes in providing tailored automated inspection solutions designed specifically for the pharmaceutical sector, helping clients meet regulatory standards and enhance product excellence.
How do automated inspection solutions benefit pharmaceutical companies?
Automated inspection solutions improve operational efficiency, reduce the risk of non-compliance, and facilitate adherence to regulatory standards such as GXP and FDA guidelines.
What is the projected growth of the pharmaceutical evaluation machines market?
The pharmaceutical evaluation machines market is projected to grow from USD 862.0 million in 2023 to USD 1.62 billion by 2030, driven by the adoption of advanced assessment technologies.
What percentage of the market share do automated inspection machines hold in 2023?
In 2023, automated inspection machines accounted for 76.0% of the market share in the pharmaceutical evaluation machines market.
How are AI and machine learning impacting automated inspection in pharmaceuticals?
AI and machine learning are enhancing evaluation capabilities by enabling real-time defect detection and improving overall efficiency in the inspection processes.
What are the key benefits of implementing automated inspection technologies in pharmaceutical companies?
Implementing automated inspection technologies leads to substantial improvements in quality assurance processes, streamlining operations, and upholding high standards of safety and efficacy in pharmaceutical products.
How do automated inspection systems contribute to regulatory compliance?
Automated inspection systems enhance regulatory compliance by ensuring adherence to Good Manufacturing Practices (GMP) and providing real-time oversight and documentation of evaluation procedures, minimizing human error.
What impact does automation have on audit findings in pharmaceutical firms?
Organizations using automated inspection systems report improved adherence outcomes, including a reduction in audit findings and enhanced overall product quality.
How much can automation accelerate evaluation processes in pharmaceutical production?
Automation can accelerate evaluation processes by up to 100 times, resulting in a 90% decrease in reporting mistakes.
What role does machine vision technology play in automated inspection?
Machine vision technology facilitates thorough evaluations of pill uniformity, ensuring that only flawless products reach consumers and enhancing compliance and productivity in the pharmaceutical sector.
